Trưởng ban: PGS.TS. PHẠM NGUYỄN VINH
Tham gia biên soạn: PGS.TS. HỒ HUỲNH QUANG TRÍ,
TS.BS. TRẦN VŨ MINH THƯ, BS CKII. LÊ THỊ ĐẸP,
BS CKII. TRẦN THỊ TUYẾT LAN, ThS.BS. HUỲNH THANH KIỀU,
ThS.BS. PHẠM ĐỖ ANH THƯ, BS CKI. PHẠM THỤC MINH THỦY
Biên tập: LƯƠNG BÍCH NHUNG, TRẦN THỊ THANH NGA
DANH MỤC TỪ VIẾT TẮT
| Từ viết tắt | Tiếng Việt | Tiếng Anh |
| ΔP | Độ chênh áp lực | – |
| AVAi | Chỉ số diện tích mở van ĐMC | |
| ASA | Aspirin | Aspirin |
| BMV | Bệnh mạch vành | – |
| CABG | Phẫu thuật bắc cầu động mạch vành | Coronary artery bypass graft surgery |
| Chụp MDCT | Chụp cắt lớp vi tính đa dãy | MDCT (Multidetector computed tomography) |
| CT | Chụp cắt lớp vi tính | Computed tomography |
| ĐMC | Động mạch chủ | – |
| ĐMP | Động mạch phổi | – |
| AVAi | Chỉ số Diện tích mở van | Aortic Valve Area (index) |
| FFR | Phân suất dự trữ lưu lượng mạch vành | Fractional flow reserve |
| iFR | Chỉ số sóng tự do tức thời | Instantaneous wave free ratio |
| INR | Tỉ lệ chuẩn hóa quốc tế | International Normalized Ratio |
| MCC | Mức chứng cứ | – |
| MDT | Nhóm đa chuyên khoa | Multi-Disciplinary Team |
| MIDA | – | Mitral Regurgitation International Database |
| MRA | Cộng hưởng từ mạch máu | Magnetic Resonance Angiography |
| MSCT | Chụp CT đa lát cắt | Multidetector Slices Computed Tomography |
| PCI | Can thiệp động mạch vành qua da | Percutaneous coronary intervention |
| PET | Chụp cắt lớp phát xạ positron | Positron emission tomography |
| PISA | – | Proximal Isovelocity Surface Area |
| PMBC | Nong van hai lá bằng bóng | Percutaneous Mitral Balloon Commissurotomy |
| PSTM | Phân suất tống máu | – |
| PSTMTT
LVEF |
Phân suất tống máu thất trái | Left Ventricular Ejection Fraction |
| LFLG | Cung lượng tim thấp | Low flow, low gradient |
| LMWH | Heparin trọng lượng phân tử thấp | Low-molecular-weight heparin |
| LVESD | Đường kính thất trái cuối tâm thu | Left Ventricle End-systolic Dimension |
| SATQTN
TTE |
Siêu âm tim qua thành ngực | Trans-Thoracic Echocardiography |
| SATQTQ
TEE |
Siêu âm tim qua thực quản | Trans-Oesophageal Echocardiography |
| SAVR | Thay van động mạch chủ bằng phẫu thuật | Surgical Aortic Valve Replacement |
| STS | Hội phẫu thuật lồng ngực | Society of thoracic surgeons |
| SYNTAX | – | Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery |
| SVI | Chỉ số cung lượng thất | Stroke volume index |
| TAVI | Thay van động mạch chủ qua da | Transcatheter Aortic Valve Implantation |
| TEER | Kẹp các bờ lá van qua đường ống thông | Transcatheter Edge-To-Edge Repair |
| TTR | Thời gian trong khoảng điều trị | Time in Therapeutic Range |
| V | Vận tốc | Velocity |
| VC | – | Vena Contracta |
| UFH | Heparin không phân đoạn | Unfractionated heparin |
| VNTMNT | Viêm nội tâm mạc nhiễm trùng | – |
Lời mở đầu
Bệnh van tim có thể là nguyên nhân hay hậu quả của tổn thương cơ tim, từ đó có bệnh van tim nguyên phát và van tim thứ phát. Nhờ các tiến bộ của lâm sàng và cận lâm sàng (siêu âm tim, chụp cắt lớp điện toán, cộng hưởng từ, thông tim), chẩn đoán bệnh van tim đã được chuẩn hóa.
Hội Tim mạch Quốc gia Việt Nam đã có khuyến cáo về bệnh van tim từ vài năm trước. Khuyến cáo này cập nhật các tiến bộ trong chẩn đoán và điều trị dựa trên các nghiên cứu mới. Khuyến cáo cũng tham khảo các khuyến cáo gần đây của Hội Tim mạch châu Âu, khuyến cáo của Hội Tim mạch Hoa Kỳ.
Mức khuyến cáo được xếp loại I có ý nghĩa cần thực hiện, lợi ích cao hơn nguy cơ. Mức IIa khi độ mạnh cũng là trung bình, dù thực hiện lợi vẫn cao hơn nguy cơ; mức IIb khi khuyến cáo thực hiện ở vị trí yếu, vẫn có lợi hoặc lợi bằng hại khi thực hiện. Mức chứng cứ loại III khi không nên thực hiện, nguy cơ cao hơn lợi ích.
Mức chứng cứ là A khi dựa vào nhiều nghiên cứu có phân phối ngẫu nhiên hoặc nghiên cứu phân tích tổng hợp. Mức B khi chỉ dựa vào một nghiên cứu ngẫu nhiên. Mức C khi dựa vào nghiên cứu quan sát sổ bộ hay đồng thuận.
Bảng 1. Phân loại các khuyến cáo

Bảng 2. Các mức chứng cứ

Nguyên tắc chung
- Bệnh van tim có thể phát hiện nhờ vào triệu chứng cơ năng, nghe được âm thổi, hoặc phát hiện tình cờ qua siêu âm tim.
- Khi nghi ngờ có bệnh van tim, cần hỏi kỹ bệnh sử, khám thực thể. Điện tâm đồ (ECG), X-quang ngực và siêu âm tim 2D và Doppler màu.
- Để có đầy đủ dữ kiện giúp chẩn đoán và điều trị, đôi khi cần siêu âm tim qua thực quản, chụp cắt lớp điện toán, ảnh cộng hưởng từ, trắc nghiệm gắng sức, Holter ECG, thông tim khảo sát huyết động và chụp cắt lớp phóng tia positron kết hợp với cắt lớp điện toán.
1. Các định nghĩa về độ nặng của bệnh van tim
Độ nặng của van dựa vào nhiều tiêu chuẩn: triệu chứng cơ năng, giải phẫu học lá van, huyết động của van và ảnh hưởng của rối loạn chức năng van trên tâm thất.
Các giai đoạn của bệnh van tim được phân chia ra A, B, C, D.
Bảng 3. Các giai đoạn của bệnh van tim (1)

2. Chẩn đoán bệnh van tim
Siêu âm tim qua thành ngực (SATQTN) là phương tiện chính chẩn đoán bệnh van tim(2–5). Một số bệnh nhân cần thêm siêu âm tim gắng sức, siêu âm tim qua thực quản (SATQTQ), thông tim và chụp cắt lớp điện toán hoặc ảnh cộng hưởng từ.
Bệnh nhân bị bệnh van tim cần được khám ngay khi xuất hiện triệu chứng cơ năng hay có thay đổi triệu chứng cơ năng. Lúc này cần làm lại SATQTN. Triệu chứng thực thể mới xuất hiện cũng cần SATQTN (2–8).
Theo dõi định kỳ rất cần thiết với bệnh nhân bệnh van tim. Tối thiểu mỗi năm cần được khám nghiệm một lần, gần hơn khi triệu chứng cơ năng gia tăng.
Khi cận lâm sàng không xâm nhập không giúp cắt nghĩa hoặc khi có bất tương hợp giữa cận lâm sàng không xâm nhập với triệu chứng cơ năng, cần khảo sát thông tim giúp đo trực tiếp áp lực buồng tiim, độ chênh áp qua van, cung lượng tim và sức cản mạch phổi.
Một số bệnh nhân bệnh van tim, trắc nghiệm gắng sức sẽ giúp có quyết định điều trị tối ưu. Khi triệu chứng cơ năng mơ hồ, trắc nghiệm gắng sức sẽ giúp phát hiện (2,3).
3. Các nguyên tắc cơ bản điều trị nội khoa
Một số nguyên tắc cơ bản trong điều trị nội khoa bệnh nhân bệnh van tim:
- Các yếu tố nguy cơ tim mạch như tăng huyết áp, tiểu đường, tăng lipid máu và lối sống lành mạnh cần được quan tâm điều trị (tập thể dục, ăn lành mạnh, không thuốc lá, giữ cân nặng bình thường).
- Thể dục vận động hay thể dục đẳng lực với tạ nhẹ đều hữu ích tăng cường lực cơ.
- Mặc dù điều trị chính bệnh lý của van là thiết yếu; một số bệnh nhân từ chối can thiệp hay không còn can thiệp được, điều trị suy tim tâm thu bằng thuốc theo khuyến cáo vẫn hữu ích.
- Các bệnh nhân có hẹp van, không nên điều trị giảm đột ngột huyết áp.
- Cần phòng ngừa thấp tim và phòng ngừa viêm nội tâm mạc nhiễm trùng. Vệ sinh răng miệng rất cần thiết trong phòng ngừa cúm mùa, giúp ngăn ngừa yếu tố làm nặng bệnh.
4. Phòng ngừa thấp tim
Phòng ngừa thấp tim thường lâu dài, nhiều năm: 5 năm, 10 năm hoặc lâu hơn đến năm 60 tuổi. tùy theo điều kiện người bệnh, có biện pháp thích hợp: thuốc tiêm hoặc thuốc uống.
Bảng 4. Phòng ngừa thứ cấp thấp tim (1)
| Kháng sinh phòng ngừa | Liều dùng* |
| Penicillin G benzathine | Tiêm bắp 1,2 triệu đơn vị/ mỗi 4 tuần † |
| Penicillin V potassium | 200mg, uống ngày 2 lần |
| Sulfadiazine | 1g uống ngày 1 lần |
| Macrolide hoặc kháng sinh Azalide (bệnh nhân dị ứng penicillin và sulfadiazine)ǂ | Liều thay đổi |
| *Bệnh nhân có bệnh van tim hậu thấp, cần phòng ngừa 10 năm hay tới 40 tuổi. Phòng ngừa suốt đời với bệnh nhân nguy cơ cao. Phòng ngừa thứ cấp cần thiết cho cả bệnh nhân đã thay van tim | |
5. Phòng ngừa viêm nội tâm mạc nhiễm trùng (VNTMNT)
Trên van đã bị tổn thương hoặc van nhân tạo, VNTMNT có thể xảy ra do vi trùng từ răng miệng hay nhiễm trùng từ ngoài da. Các bệnh tim bẩm sinh chưa được điều trị cũng có nguy cơ VNTMNT.
| Loại | MCC | Khuyến cáo phòng ngừa VNTMNT(1) |
| IIa | C | 1. Kháng sinh phòng ngừa cần thiết cho mọi thủ thuật răng miệng, trên bệnh nhân có bệnh van tim có kèm:(9)
a. Van nhân tạo, sau phẫu thuật hay thay qua da b. Vật liệu nhân tạo sửa can, bao gồm vòng van, dây chằng, clips c. Tiền sử VNTMNT d. Bệnh tim bẩm sinh tím không điều trị hoặc đã điều trị nhưng có shunt tồn lưu hoặc hở van e. Van hở trên tim ghép |
| III
không có lợi |
B | 2. Bệnh nhân có bệnh van tim và có nguy cơ cao VNTMNT, kháng sinh phòng ngừa không được khuyến cáo cho thủ thuật ngoài răng (VD: siêu âm tim qua thực quản, nội soi thực quản ruột non, nội soi dạ dàym nội soi bàng quang), nếu không có nhiều bằng cấp(10,11). |
VNTMNT này là nguy cơ của van được ghép qua thông tim (ví dụ: TAVI). Tim được cấy ghép, đặc biệt trong 6 tháng đầu, có nguy cơ cao VNTMNT.
Vệ sinh răng miệng rất cần thiết. Sử dụng bàn chải đánh răng hay kéo chỉ có thể tăng 20% đến 68% vi trùng xâm nhập máu, sử dụng tăm cũng dẫn đến hiện tượng tương tự từ 20 – 40%. Do đó giữ cho bộ răng không bị bệnh rất cần thiết.
6. Kháng đông điều trị rung nhĩ kèm bệnh van tim
| Loại | MCC | Khuyến cáo về dùng kháng đông điều trị rung nhĩ kèm bệnh van tim (1) |
| I | C | 1. Bệnh nhân có rung nhĩ và bệnh van tự nhiên, ngoại trừ hẹp 2 lá hậu thấp hoặc bệnh nhân có van sinh học > 3 tháng, kháng đông mới (NOAC) có thể thay thế thuốc kháng vitamin K. Cần cho theo chỉ định CHA2DS2-VAS score.(12,13) |
| I | A | 2. Rung nhĩ và hẹp 2 lá hậu thấp, kháng vitamin K uống cần lâu dài |
| IIa | B | 3. Bệnh nhân mới rung nhĩ khoảng 3 tháng sau phẫu thuật hoặc thay van sinh học qua da, kháng đông với kháng vitamin K là hợp lý(14–16) |
| III
có hại |
B | 4. Không sử dụng NOAC ở bệnh nhân van cơ học dù có hay không rung nhĩ (17) |
Kháng vitamin K (warfarin hay acenocoumarol) cần được sử dụng phòng ngừa huyết khối cho bệnh nhân hẹp van hai lá hậu thấp hoặc bệnh nhân có van tim cơ học. Kháng đông mới (NOAC) có thể thay thế kháng vitamin K ở bệnh nhân rung nhĩ có van sinh học sau cấy ghép 3 tháng hoặc van tim tự nhiên bị bệnh (ngoại trừ hẹp hai hai lá do thấp tim).
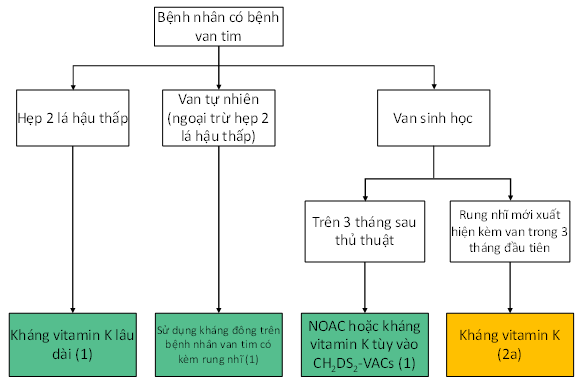
Hình 1. Sử dụng kháng đông trên bệnh nhân van tim có kèm rung nhĩ (1)
Rung nhĩ xảy ra sau phẫu thuật van tim làm tăng tật bệnh và tử vong (14,15) bất kể điểm CHA₂DS₂-VASc. Do đó những bệnh nhân này cần được sử dụng thuốc kháng vitamin K trong 3 tháng đầu. Sau đó lượng định lại, tùy theo điểm CHA₂DS₂-VASc có thể sử dụng kháng đông mới.
7. Khảo sát nguy cơ can thiệp và nguy cơ phẫu thuật
Khảo sát nguy cơ trước phẫu thuật hay can thiệp thay van qua ống thông rất cần thiết. Có thể sử dụng các ứng dụng trực tuyến cần bàn luận trước thủ thuật giữa bác sĩ Nội tim mạch, phẫu thuật viên và bác sĩ can thiệp. Chia sẻ quyết định điều trị với bệnh nhân cũng rất hữu ích.
Bảng 5. Nguy cơ tử vong của các phẫu thuật đặc biệt (Theo chỉ số STS 2019) (1)
| Thủ thuật | Tỷ lệ tử vong (%) |
| Thay van động mạch chủ | 2.2 |
| Thay van ĐMC và BCĐMV | 4 |
| Thay van ĐMC và van 2 lá | 9 |
| Thay van 2 lá | 5 |
| Thay van 2 lá và BCĐMV | 9 |
| Sửa van 2 lá | 1 |
| Sửa van 2 lá và bắc cầu ĐMV | 5 |
| BCĐMV = Bắc cầu động mạch vành; STS = Society of Thoracic Surgeons. | |
8. Xử trí bệnh nhân bệnh van tim sau can thiệp sửa hay thay van
Tất cả các bệnh nhân được điều trị can thiệp van (thay van qua da, sửa van, thay van bằng phẫu thuật) cần được chăm sóc và theo dõi định kỳ suốt đời người bệnh: điều trị nội khoa, theo dõi van nhân tạo hay van được sửa (lâm sàng, siêu âm tim,…), phòng viêm nội tâm mạc nhiễm trùng, sử dụng thuốc kháng đông thích hợp, điều trị các biến chứng và các bệnh đồng mắc.
8.1. Các biến chứng sau thủ thuật
Rung nhĩ chiếm 1/3 trong vòng 3 tháng sau phẫu thuật thay van. Các biến chứng khác bao gồm: đột quỵ, biến chứng xuất huyết, viêm màng ngoài tim, blốc tim có thể cần tạo nhịp tạm thời hay vĩnh viễn (thường gặp sau thay van động mạch chủ), suy tim, rối loạn chức năng thận và nhiễm trùng.
8.2. Triệu chứng cơ năng kéo dài sau can thiệp van
Triệu chứng cơ năng kéo dài có thể xảy ra ở nhiều bệnh nhân sau can thiệp van. Cần thực hiện các bước sau nhằm tìm nguyên nhân: khảo sát lại chức năng van khảo sát và điều trị các bệnh đồng mắc do tim và không do tim. Điều trị các biểu hiện này theo đúng khuyến cáo.
9. Hẹp van động mạch chủ
9.1. Các giai đoạn bệnh
Diện tích mở van (DTMV) động mạch chủ (ĐMC) bình thường từ 3 – 5 cm2, và độ chênh áp từ 4 – 6 mmHg. Khi DTMV giảm 50%, bắt đầu có thay đổi huyết động ngang van và độ chênh áp lực tăng. DTMV giảm còn 30% bình thường (1 cm2), chênh áp lực trung bình ngang van trên 40 mmHg, gọi là hẹp van ĐMC nặng.
Nguyên nhân của hẹp van ĐMC thường gặp gồm bệnh van hậu thấp (14% – 35%), thoái hóa vôi ở người lớn tuổi (trên 65 tuổi), bẩm sinh (ví dụ: van ĐMC hai mảnh).
Theo Hiệp hội Tim Mạch Hoa Kỳ, hẹp van ĐMC được chia làm bốn giai đoạn A, B, C và D để có hướng theo dõi và can thiệp kịp thời(1).
Giai đoạn A: bệnh nhân có nguy cơ hẹp van động mạch chủ như người có bất thường bẩm sinh van ĐMC hai mảnh hoặc 1 mảnh, van động mạch chủ sợi hóa, xơ hóa ở người lớn. Trong giai đoạn này người bệnh cần được theo dõi định kỳ và điều chỉnh các yếu tố nguy cơ dẫn đến hẹp van ĐMC tiến triển.
Giai đoạn B: hẹp van ĐMC tiến triển, bao gồm bệnh van ĐMC bẩm sinh hai mảnh có vôi hóa, sợi hóa lá van tiến triển; hoặc bệnh van tim hậu thấp có thay đổi trên van ĐMC. Trong giai đoạn này, người bệnh có hẹp van ĐMC mức độ nhẹ đến trung bình, có thay đổi huyết động học ngang van. Vận tốc dòng máu ngang van ĐMC tăng trên 2 m/giây, nhưng dưới 4 m/giây, chênh áp lực trung bình ngang van dưới 40 mmHg. Bệnh nhân không có triệu chứng lâm sàng trong giai đoạn này. Người bệnh cần được theo dõi sát tiến triển của bệnh, điều chỉnh các yếu tố nguy cơ, điều trị phòng thấp nếu có thấp tim.
Giai đoạn C: hẹp van ĐMC nặng không có triệu chứng cơ năng với vận tốc dòng máu ngang van ĐMC ≥ 4 m/giây, chênh áp trung bình ≥ 40 mmHg, và diện tích mở van dưới 1.0 cm2 (hoặc AVAi dưới 0.6 cm2). Trong giai đoạn C được chia ra C1 là hẹp van ĐMC nặng không triệu chứng và hẹp rất nặng khi vận tốc ≥ 5 m/giây và chênh áp trung bình ≥ 60 mmHg, C2 là hẹp van ĐMC nặng không triệu chứng với PSTMTT giảm dưới 50%.
Giai đoạn D: hẹp van ĐMC nặng có triệu chứng như khó thở, đau thắt ngực, ngất hoặc gần ngất khi gắng sức hoặc suy tim. Trong giai đoạn D gồm D1, D2 và D3.
- Giai đoạn D1: hẹp van ĐMC nặng có triệu chứng cơ năng, chênh áp lực ngang van cao với chênh áp trung bình ≥ 40 mmHg, DTMV ≤ 1.0 cm2 (hoặc AVAi ≤ 0.6 cm2/m2), có thể lớn hơn nếu hẹp hở van kết hợp.
- Giai đoạn D2: hẹp van ĐMC nặng có triệu chứng cơ năng, chênh áp lực ngang van thấp với chênh áp trung bình dưới 40 mmHg, PSTMTT giảm dưới 50%. DTMV theo phương trình liên tục đo được ≤1 cm2. Trường hợp này bệnh nhân cần được làm thêm siêu âm tim gắng sức dobutamine liều thấp để đánh giá độ nặng thật sự của hẹp van ĐMC. Nếu lúc làm siêu âm gắng sức, DTMV vẫn ≤1 cm2và Vmax tăng lên ≥ 4 m/giây thì người bệnh có hẹp van ĐMC nặng thật sự.
- Giai đoạn D3: hẹp van ĐMC nặng có triệu chứng, chênh áp ngang van thấp với phân suất tống máu bình thường hoặc hẹp van ĐMC nặng cung lượng thấp nghịch thường. Khi đó chênh áp trung bình ngang van dưới 40 mmHg, chỉ số cung lượng thất (Stroke volume index) dưới 35 ml/m2 và PSTMTT ≥ 50% và điều kiện khi làm siêu âm tim là huyết áp tâm thu dưới 140 mmHg. Tình trạng này thường gặp ở người bệnh có thành thất trái dày nhiều, buồng thất trái nhỏ, thể tích nhát bóp giảm.
(Còn nữa…)
TÀI LIỆU THAM KHẢO
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25-e197. https://doi.org/10.1016/j.jacc.2020.11.018
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22(1):1-23. https://doi.org/10.1016/J.ECHO.2008.11.029
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010;11(4):307-332. https://doi.org/10.1093/EJECHOCARD/JEQ031
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303-371. https://doi.org/10.1016/J.ECHO.2017.01.007
- Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(4):372-392. https://doi.org/10.1016/J.ECHO.2017.02.009
- Currie PJ, Seward JB, Reeder GS, et al. Continuous-wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous Doppler-catheter correlative study in 100 adult patients. Circulation. 1985;71(6):1162-1169. https://doi.org/10.1161/01.CIR.71.6.1162
- Medvedofsky D, Maffessanti F, Weinert L, et al. 2D and 3D Echocardiography-Derived Indices of Left Ventricular Function and Shape: Relationship With Mortality. JACC Cardiovasc Imaging. 2018;11(11):1569-1579. https://doi.org/10.1016/J.JCMG.2017.08.023
- Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167-184. https://doi.org/10.1067/MJE.2002.120202
- Habib G, Lancellotti P. The 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J. 2015;36(44):3036-3037. https://doi.org/10.1093/EURHEARTJ/EHV488
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111(24):3290-3295. https://doi.org/10.1161/CIRCULATIONAHA.104.495903
- Rosenhek R, Iung B, Tornos P, et al. ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J. 2012;33(7). https://doi.org/10.1093/EURHEARTJ/EHR061
- Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6(7). https://doi.org/10.1161/JAHA.117.005835
- Lip GYH, Jensen M, Melgaard L, Skjøth F, Nielsen PB, Larsen TB. Stroke and bleeding risk scores in patients with atrial fibrillation and valvular heart disease: evaluating “valvular heart disease” in a nationwide cohort study. Europace. 2019;21(1):33-40. https://doi.org/10.1093/EUROPACE/EUY151
- Vora AN, Dai D, Matsuoka R, et al. Incidence, Management, and Associated Clinical Outcomes of New-Onset Atrial Fibrillation Following Transcatheter Aortic Valve Replacement: An Analysis From the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2018;11(17):1746-1756. https://doi.org/10.1016/J.JCIN.2018.05.042
- Seeger J, Gonska B, Rodewald C, Rottbauer W, Wöhrle J. Apixaban in Patients With Atrial Fibrillation After Transfemoral Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10(1):66-74. https://doi.org/10.1016/J.JCIN.2016.10.023
- Jochheim D, Barbanti M, Capretti G, et al. Oral Anticoagulant Type and Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12(16):1566-1576. https://doi.org/10.1016/J.JCIN.2019.03.003
- JW E, SJ C, M B, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13). https://doi.org/10.1056/NEJMOA1300615
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561-632. https://doi.org/10.1093/eurheartj/ehab395
- Aicher D, Kunihara T, Abou Issa O, Brittner B, Gräber S, Schäfers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123(2):178-185. https://doi.org/10.1161/CIRCULATIONAHA.109.934679
- David TE, Armstrong S, Ivanov J, Webb GD. Aortic valve sparing operations: an update. Ann Thorac Surg. 1999;67(6):1840-1842. https://doi.org/10.1016/S0003-4975(99)00420-8
- Kallenbach K, Hagl C, Walles T, et al. Results of valve-sparing aortic root reconstruction in 158 consecutive patients. Ann Thorac Surg. 2002;74(6):2026-2033. https://doi.org/10.1016/S0003-4975(02)04090-0
- Pettersson GB, Crucean AC, Savage R, et al. Toward Predictable Repair of Regurgitant Aortic Valves: A Systematic Morphology-Directed Approach to Bicommissural Repair. J Am Coll Cardiol. 2008;52(1):40-49. https://doi.org/10.1016/J.JACC.2008.01.073
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg. 2011;142(6):1430-1438. https://doi.org/10.1016/J.JTCVS.2011.08.021
- Kari FA, Liang DH, Escobar Kvitting JP, et al. Tirone David valve-sparing aortic root replacement and cusp repair for bicuspid aortic valve disease. J Thorac Cardiovasc Surg. 2013;145(3):S35-S40.e2. https://doi.org/10.1016/J.JTCVS.2012.11.043
- Ouzounian M, Rao V, Manlhiot C, et al. Valve-Sparing Root Replacement Compared With Composite Valve Graft Procedures in Patients With Aortic Root Dilation. J Am Coll Cardiol. 2016;68(17):1838-1847. https://doi.org/10.1016/J.JACC.2016.07.767
- Lang RM, Badano LP, Victor MA, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2015;28(1):1-39.e14. https://doi.org/10.1016/J.ECHO.2014.10.003
- Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. Journal of Cardiovascular Magnetic Resonance. 2015;17(1):1-33. https://doi.org/10.1186/S12968-015-0111-7/COMMENTS
- Bonow RO, Borer JS, Rosing DR, et al. Preoperative exercise capacity in symptomatic patients with aortic regurgitation as a predictor of postoperative left ventricular function and long-term prognosis. Circulation. 1980;62(6):1280-1290. https://doi.org/10.1161/01.CIR.62.6.1280
- Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Optimizing Timing of Surgical Correction in Patients With Severe Aortic Regurgitation: Role of Symptoms. J Am Coll Cardiol. 1997;30(3):746-752. https://doi.org/10.1016/S0735-1097(97)00205-2
- Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002;106(21):2687-2693. https://doi.org/10.1161/01.CIR.0000038498.59829.38
- Tornos P, Sambola A, Permanyer-Miralda G, Evangelista A, Gomez Z, Soler-Soler J. Long-Term Outcome of Surgically Treated Aortic Regurgitation: Influence of Guideline Adherence Toward Early Surgery. J Am Coll Cardiol. 2006;47(5):1012-1017. https://doi.org/10.1016/J.JACC.2005.10.049
- Bhudia SK, McCarthy PM, Kumpati GS, et al. Improved Outcomes After Aortic Valve Surgery for Chronic Aortic Regurgitation With Severe Left Ventricular Dysfunction. J Am Coll Cardiol. 2007;49(13):1465-1471. https://doi.org/10.1016/J.JACC.2007.01.026
- Fiedler AG, Bhambhani V, Laikhter E, et al. Aortic valve replacement associated with survival in severe regurgitation and low ejection fraction. Heart. 2018;104(10):835-840. https://doi.org/10.1136/HEARTJNL-2017-312024
- Kaneko T, Ejiofor JI, Neely RC, et al. Aortic Regurgitation With Markedly Reduced Left Ventricular Function Is Not a Contraindication for Aortic Valve Replacement. Ann Thorac Surg. 2016;102(1):41-47. https://doi.org/10.1016/J.ATHORACSUR.2015.12.068
- Forman R, Firth BG, Barnard MS. Prognostic significance of preoperative left ventricular ejection fraction and valve lesion in patients with aortic valve replacement. Am J Cardiol. 1980;45(6):1120-1125. https://doi.org/10.1016/0002-9149(80)90468-3
- Bonow RO, Picone AL, McIntosh CL, et al. Survival and functional results after valve replacement for aortic regurgitation from 1976 to 1983: impact of preoperative left ventricular function. Circulation. 1985;72(6):1244-1256. https://doi.org/10.1161/01.CIR.72.6.1244
- Cormier B, Vahanian A, Luxereau P, Kassab R, Acar J. Should asymptomatic or mildly symptomatic aortic regurgitation be operated on? Z Kardiol. 1986;75:141-145.
- Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Aortic regurgitation complicated by extreme left ventricular dilation: long-term outcome after surgical correction. J Am Coll Cardiol. 1996;27(3):670-677. https://doi.org/10.1016/0735-1097(95)00525-0
- Saisho H, Arinaga K, Kikusaki S, et al. Long Term Results and Predictors of Left Ventricular Function Recovery after Aortic Valve Replacement for Chronic Aortic Regurgitation. Annals of Thoracic and Cardiovascular Surgery. 2015;21(4):388-395. https://doi.org/10.5761/ATCS.OA.14-00295
- Mentias A, Feng K, Alashi A, et al. Long-Term Outcomes in Patients With Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2016;68(20):2144-2153. https://doi.org/10.1016/J.JACC.2016.08.045
- Yang LT, Michelena HI, Scott CG, et al. Outcomes in Chronic Hemodynamically Significant Aortic Regurgitation and Limitations of Current Guidelines. J Am Coll Cardiol. 2019;73(14):1741-1752. https://doi.org/10.1016/J.JACC.2019.01.024
- de Meester C, Gerber BL, Vancraeynest D, et al. Do Guideline-Based Indications Result in an Outcome Penalty for Patients With Severe Aortic Regurgitation? JACC Cardiovasc Imaging. 2019;12(11):2126-2138. https://doi.org/10.1016/J.JCMG.2018.11.022
- Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84(4):1625-1635. https://doi.org/10.1161/01.CIR.84.4.1625
- Pizarro R, Bazzino OO, Oberti PF, et al. Prospective Validation of the Prognostic Usefulness of B-Type Natriuretic Peptide in Asymptomatic Patients With Chronic Severe Aortic Regurgitation. J Am Coll Cardiol. 2011;58(16):1705-1714. https://doi.org/10.1016/J.JACC.2011.07.016
- Tornos MP, Olona M, Permanyer-Miralda G, et al. Clinical outcome of severe asymptomatic chronic aortic regurgitation: A long-term prospective follow-up study. Am Heart J. 1995;130(2):333-339. https://doi.org/10.1016/0002-8703(95)90450-6
- Tarasoutchi F, Grinberg M, Spina GS, et al. Ten-year clinical laboratory follow-up after application of a symptom-based therapeutic strategy to patients with severe chronic aortic regurgitation of predominant rheumatic etiology. J Am Coll Cardiol. 2003;41(8):1316-1324. https://doi.org/10.1016/S0735-1097(03)00129-3
- Kumpuris AG, Quinones MA, Waggoner AD, Kanon DJ, Nelson JG, Miller RR. Importance of preoperative hypertrophy, wall stress and end-systolic dimension as echocardiographic predictors of normalization of left ventricular dilatation after valve replacement in chronic aortic insufficiency. Am J Cardiol. 1982;49(5):1091-1100. https://doi.org/10.1016/0002-9149(82)90032-7
- Fioretti P, Roelandt J, Bos RJ, et al. Echocardiography in chronic aortic insufficiency. Is valve replacement too late when left ventricular end-systolic dimension reaches 55 mm? Circulation. 1983;67(1):216-221. https://doi.org/10.1161/01.CIR.67.1.216
- Detaint D, Messika-Zeitoun D, Maalouf J, et al. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. JACC Cardiovasc Imaging. 2008;1(1):1-11. https://doi.org/10.1016/J.JCMG.2007.10.008
- Stone PH, Clark RD, Goldschlager N, Selzer A, Cohn K. Determinants of prognosis of patients with aortic regurgitation who undergo aortic valve replacement. J Am Coll Cardiol. 1984;3(5):1118-1126. https://doi.org/10.1016/S0735-1097(84)80168-0
- Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Aortic regurgitation complicated by extreme left ventricular dilation: Long-term outcome after surgical correction. J Am Coll Cardiol. 1996;27(3):670-677. https://doi.org/10.1016/0735-1097(95)00525-0
- Zhang Z, Yang J, Yu Y, et al. Preoperative ejection fraction determines early recovery of left ventricular end-diastolic dimension after aortic valve replacement for chronic severe aortic regurgitation. Journal of Surgical Research. 2015;196(1):49-55. https://doi.org/10.1016/J.JSS.2015.02.069
- Murashita T, Schaff H v., Suri RM, et al. Impact of Left Ventricular Systolic Function on Outcome of Correction of Chronic Severe Aortic Valve Regurgitation: Implications for Timing of Surgical Intervention. Ann Thorac Surg. 2017;103(4):1222-1228. https://doi.org/10.1016/J.ATHORACSUR.2016.09.004
- Wang Y, Jiang W, Liu J, et al. Early surgery versus conventional treatment for asymptomatic severe aortic regurgitation with normal ejection fraction and left ventricular dilatation. Eur J Cardiothorac Surg. 2017;52(1):118-124. https://doi.org/10.1093/EJCTS/EZX018
- Scognamiglio R, Rahimtoola SH, Fasoli G, Nistri S, Volta SD. Nifedipine in asymptomatic patients with severe aortic regurgitation and normal left ventricular function. N Engl J Med. 1994;331(11):689-694. https://doi.org/10.1056/NEJM199409153311101
- Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart. 2017;103(17):1323-1330. https://doi.org/10.1136/HEARTJNL-2016-309916
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44(1):138-143. https://doi.org/10.1016/J.JACC.2004.03.050
- Huntington K, Hunter AGW, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol. 1997;30(7):1809-1812. https://doi.org/10.1016/S0735-1097(97)00372-0
- Glick BN, Roberts WC. Congenitally bicuspid aortic valve in multiple family members. Am J Cardiol. 1994;73(5):400-404. https://doi.org/10.1016/0002-9149(94)90018-3
- Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: A disorder of uncertain inheritance. Am J Med Genet. 1996;62(4):336-338. https://doi.org/10.1002/(SICI)1096-8628(19960424)62:4<336::AID-AJMG2>3.0.CO;2-P
- Kong WKF, Delgado V, Bax JJ. Bicuspid Aortic Valve: What to Image in Patients Considered for Transcatheter Aortic Valve Replacement? Circ Cardiovasc Imaging. 2017;10(9). https://doi.org/10.1161/CIRCIMAGING.117.005987
- Michelena HI, Prakash SK, Corte A della, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 2014;129(25):2691-2704. https://doi.org/10.1161/CIRCULATIONAHA.113.007851
- Elefteriades JA, Sang A, Kuzmik G, Hornick M. Guilt by association: paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart. 2015;2(1). https://doi.org/10.1136/OPENHRT-2014-000169
- Burstow DJ, Nishimura RA, Bailey KR, et al. Continuous wave Doppler echocardiographic measurement of prosthetic valve gradients. A simultaneous Doppler-catheter correlative study. Circulation. 1989;80(3):504-514. https://doi.org/10.1161/01.CIR.80.3.504
- Dumesnil JG, Honos GN, Lemieux M, Beauchemin J. Validation and applications of indexed aortic prosthetic valve areas calculated by Doppler echocardiography. J Am Coll Cardiol. 1990;16(3):637-643. https://doi.org/10.1016/0735-1097(90)90355-S
- Baumgartner H, Khan S, DeRobertis M, Czer L, Maurer G. Effect of prosthetic aortic valve design on the Doppler-catheter gradient correlation: an in vitro study of normal St. Jude, Medtronic-Hall, Starr-Edwards and Hancock valves. J Am Coll Cardiol. 1992;19(2):324-332. https://doi.org/10.1016/0735-1097(92)90486-7
- Vandervoort PM, Greenberg NL, Pu M, Powell KA, Cosgrove DM, Thomas JD. Pressure recovery in bileaflet heart valve prostheses. Localized high velocities and gradients in central and side orifices with implications for Doppler-catheter gradient relation in aortic and mitral position. Circulation. 1995;92(12):3464-3472. https://doi.org/10.1161/01.CIR.92.12.3464
- Salaun E, Mahjoub H, Girerd N, et al. Rate, Timing, Correlates, and Outcomes of Hemodynamic Valve Deterioration After Bioprosthetic Surgical Aortic Valve Replacement. Circulation. 2018;138(10):971-985. https://doi.org/10.1161/CIRCULATIONAHA.118.035150
- Rahimtoola SH. Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. 2010;55(22):2413-2426. https://doi.org/10.1016/J.JACC.2009.10.085
- van Geldorp MWA, Eric Jamieson WR, Kappetein AP, et al. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137(4). https://doi.org/10.1016/J.JTCVS.2008.09.028
- Salaun E, Mahjoub H, Dahou A, et al. Hemodynamic Deterioration of Surgically Implanted Bioprosthetic Aortic Valves. J Am Coll Cardiol. 2018;72(3):241-251. https://doi.org/10.1016/J.JACC.2018.04.064
- Douglas PS, Leon MB, Mack MJ, et al. Longitudinal Hemodynamics of Transcatheter and Surgical Aortic Valves in the PARTNER Trial. JAMA Cardiol. 2017;2(11):1197-1206. https://doi.org/10.1001/JAMACARDIO.2017.3306
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477-2484. https://doi.org/10.1016/S0140-6736(15)60308-7
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2485-2491. https://doi.org/10.1016/S0140-6736(15)60290-2
- Fernández-Santos S, Théron A, Pibarot P, et al. Valve hemodynamic performance and myocardial strain after implantation of a third-generation, balloon-expandable, transcatheter aortic valve. Cardiol J. 2020;27(6):789-796. https://doi.org/10.5603/CJ.A2019.0049
- Manoharan G, van Mieghem NM, Windecker S, et al. 1-Year Outcomes With the Evolut R Self-Expanding Transcatheter Aortic Valve: From the International FORWARD Study. JACC Cardiovasc Interv. 2018;11(22):2326-2334. https://doi.org/10.1016/J.JCIN.2018.07.032
- Gleason TG, Reardon MJ, Popma JJ, et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J Am Coll Cardiol. 2018;72(22):2687-2696. https://doi.org/10.1016/J.JACC.2018.08.2146
- Blackman DJ, Saraf S, MacCarthy PA, et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses. J Am Coll Cardiol. 2019;73(5):537-545. https://doi.org/10.1016/J.JACC.2018.10.078
- Søndergaard L, Ihlemann N, Capodanno D, et al. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J Am Coll Cardiol. 2019;73(5):546-553. https://doi.org/10.1016/J.JACC.2018.10.083
- Kaneko T, Aranki S, Javed Q, et al. Mechanical versus bioprosthetic mitral valve replacement in patients. J Thorac Cardiovasc Surg. 2014;147(1):117-126. https://doi.org/10.1016/J.JTCVS.2013.08.028
- Bourguignon T, Bouquiaux-Stablo AL, Loardi C, et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg. 2014;148(5):2004-2011.e1. https://doi.org/10.1016/J.JTCVS.2014.02.050
- Weber A, Noureddine H, Englberger L, et al. Ten-year comparison of pericardial tissue valves versus mechanical prostheses for aortic valve replacement in patients younger than 60 years of age. J Thorac Cardiovasc Surg. 2012;144(5):1075-1083. https://doi.org/10.1016/J.JTCVS.2012.01.024
- Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36(4):1152-1158. https://doi.org/10.1016/S0735-1097(00)00834-2
- Chan V, Jamieson WRE, Germann E, et al. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after aortic valve replacement. J Thorac Cardiovasc Surg. 2006;131(6):1267-1273. https://doi.org/10.1016/J.JTCVS.2005.11.052
- Banbury MK, Cosgrove DM, Thomas JD, et al. Hemodynamic stability during 17 years of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg. 2002;73(5):1460-1465. https://doi.org/10.1016/S0003-4975(02)03445-8
- Dellgren G, David TE, Raanani E, Armstrong S, Ivanov J, Rakowski H. Late hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2002;124(1):146-154. https://doi.org/10.1067/MTC.2002.121672
- Borger MA, Ivanov J. Twenty-Year Results of the Hancock II Bioprosthesis.; 2006. https://www.researchgate.net/publication/7297016
- Mykén PSU, Bech-Hansen O. A 20-year experience of 1712 patients with the Biocor porcine bioprosthesis. J Thorac Cardiovasc Surg. 2009;137(1):76-81. https://doi.org/10.1016/J.JTCVS.2008.05.068
- Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N Engl J Med. 2017;377(19):1847-1857. https://doi.org/10.1056/NEJMOA1613792
- Badhwar V, Ofenloch JC, Rovin JD, van Gelder HM, Jacobs JP. Noninferiority of closely monitored mechanical valves to bioprostheses overshadowed by early mortality benefit in younger patients. Ann Thorac Surg. 2012;93(3):748-753. https://doi.org/10.1016/J.ATHORACSUR.2011.12.032
- Brown ML, Schaff H v., Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg. 2008;135(4):878-884. https://doi.org/10.1016/J.JTCVS.2007.10.065
- Kulik A, Bédard P, Lam BK, et al. Mechanical versus bioprosthetic valve replacement in middle-aged patients. Eur J Cardiothorac Surg. 2006;30(3):485-491. https://doi.org/10.1016/J.EJCTS.2006.06.013
- Glaser N, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50-69 years. Eur Heart J. 2016;37(34):2658-2667. https://doi.org/10.1093/EURHEARTJ/EHV580
- Chikwe J, Chiang YP, Egorova NN, Itagaki S, Adams DH. Survival and outcomes following bioprosthetic vs mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA. 2015;313(14):1435-1442. https://doi.org/10.1001/JAMA.2015.3164
- McClure RS, McGurk S, Cevasco M, et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis. J Thorac Cardiovasc Surg. 2014;148(5):1931-1939. https://doi.org/10.1016/J.JTCVS.2013.12.042
- Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014;312(13):1323-1329. https://doi.org/10.1001/JAMA.2014.12679
- Buratto E, Shi WY, Wynne R, et al. Improved Survival After the Ross Procedure Compared With Mechanical Aortic Valve Replacement. J Am Coll Cardiol. 2018;71(12):1337-1344. https://doi.org/10.1016/J.JACC.2018.01.048
- El-Hamamsy I, Eryigit Z, Stevens LM, et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376(9740):524-531. https://doi.org/10.1016/S0140-6736(10)60828-8
- Martin E, Mohammadi S, Jacques F, et al. Clinical Outcomes Following the Ross Procedure in Adults: A 25-Year Longitudinal Study. J Am Coll Cardiol. 2017;70(15):1890-1899. https://doi.org/10.1016/J.JACC.2017.08.030
- Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJM, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333(1):11-17. https://doi.org/10.1056/NEJM199507063330103
- Sun JC, Davidson MJ, Lamy A, Eikelboom JW. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009;374(9689):565-576. https://doi.org/10.1016/S0140-6736(09)60780-7
- van de Werf F, Brueckmann M, Connolly SJ, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J. 2012;163(6). https://doi.org/10.1016/J.AHJ.2012.03.011
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89(2):635-641. https://doi.org/10.1161/01.CIR.89.2.635
- Torella M, Torella D, Chiodini P, et al. LOWERing the INtensity of oral anticoaGulant Therapy in patients with bileaflet mechanical aortic valve replacement: results from the “LOWERING-IT” Trial. Am Heart J. 2010;160(1):171-178. https://doi.org/10.1016/J.AHJ.2010.05.005
- Hering D, Piper C, Bergemann R, et al. Thromboembolic and bleeding complications following St. Jude Medical valve replacement: results of the German Experience With Low-Intensity Anticoagulation Study. Chest. 2005;127(1):53-59. https://doi.org/10.1378/CHEST.127.1.53
- Acar J, Iung B, Boissel JP, et al. AREVA: multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation. 1996;94(9):2107-2112. https://doi.org/10.1161/01.CIR.94.9.2107
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e576S-e600S. https://doi.org/10.1378/CHEST.11-2305
- Horstkotte D, Scharf RE, Schultheiss HP. Intracardiac thrombosis: patient-related and device-related factors. J Heart Valve Dis. 1995;4(2):114-120. Accessed May 30, 2022. https://europepmc.org/article/med/8556170
- Pruefer D, Dahm M, Dohmen G, Horstkotte D, Bergemann R, Oelert H. Intensity of oral anticoagulation after implantation of St. Jude Medical mitral or multiple valve replacement: lessons learned from GELIA (GELIA 5). European Heart Journal Supplements. 2001;3(suppl_Q):Q39-Q43. https://doi.org/10.1016/S1520-765X(01)90041-0
- Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc Interv. 2017;10(13):1357-1365. https://doi.org/10.1016/J.JCIN.2017.04.014
- Zuo W, Yang M, He Y, Hao C, Chen L, Ma G. Single or dual antiplatelet therapy after transcatheter aortic valve replacement: an updated systemic review and meta-analysis. J Thorac Dis. 2019;11(3):959-968. https://doi.org/10.21037/JTD.2019.01.87
- Maes F, Stabile E, Ussia GP, et al. Meta-Analysis Comparing Single Versus Dual Antiplatelet Therapy Following Transcatheter Aortic Valve Implantation. Am J Cardiol. 2018;122(2):310-315. https://doi.org/10.1016/J.AMJCARD.2018.04.006
- Heras M, Chesebro JH, Fuster V, et al. High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol. 1995;25(5):1111-1119. https://doi.org/10.1016/0735-1097(94)00563-6
- Colli A, Castella M. Comparing Warfarin to Aspirin (WoA) after Aortic Valve Replacement with the St. Jude Medical EpicTM Heart Valve Bioprosthesis: Results of the WoA Epic Pilot Trial. Published online 2007. Accessed May 31, 2022. https://www.researchgate.net/publication/5752586
- Aramendi JI, Mestres CA, Martinez-León J, Campos V, Muñoz G, Navas C. Triflusal versus oral anticoagulation for primary prevention of thromboembolism after bioprosthetic valve replacement (trac): prospective, randomized, co-operative trial. Eur J Cardiothorac Surg. 2005;27(5):854-860. https://doi.org/10.1016/J.EJCTS.2004.12.064
- Nuñez L, Aguado MG, Larrea JL, Celemín D, Oliver J. Prevention of thromboembolism using aspirin after mitral valve replacement with porcine bioprosthesis. Ann Thorac Surg. 1984;37(1):84-87. https://doi.org/10.1016/S0003-4975(10)60717-5
- Tiede DJ, Nishimura RA, Gastineau DA, Mullany CJ, Orszulak TA, Schaff H v. Modern management of prosthetic valve anticoagulation. Mayo Clin Proc. 1998;73(7):665-680. https://doi.org/10.1016/S0025-6196(11)64893-3
- Mérie C, Køber L, Skov Olsen P, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA. 2012;308(20):2118-2125. https://doi.org/10.1001/JAMA.2012.54506
- Russo A, Grigioni F, Avierinos JF, et al. Thromboembolic complications after surgical correction of mitral regurgitation incidence, predictors, and clinical implications. J Am Coll Cardiol. 2008;51(12):1203-1211. https://doi.org/10.1016/J.JACC.2007.10.058
- Egbe AC, Pislaru S v., Pellikka PA, et al. Bioprosthetic Valve Thrombosis Versus Structural Failure: Clinical and Echocardiographic Predictors. J Am Coll Cardiol. 2015;66(21):2285-2294. https://doi.org/10.1016/J.JACC.2015.09.022
- Sundt TM, Zehr KJ, Dearani JA, et al. Is early anticoagulation with warfarin necessary after bioprosthetic aortic valve replacement? J Thorac Cardiovasc Surg. 2005;129(5):1024-1031. https://doi.org/10.1016/J.JTCVS.2004.11.028
- ElBardissi AW, DiBardino DJ, Chen FY, Yamashita MH, Cohn LH. Is early antithrombotic therapy necessary in patients with bioprosthetic aortic valves in normal sinus rhythm? J Thorac Cardiovasc Surg. 2010;139(5):1137-1145. https://doi.org/10.1016/J.JTCVS.2009.10.064
- Massel DR, Little SH. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2013;2013(7). https://doi.org/10.1002/14651858.CD003464.PUB2
- Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014;147(4). https://doi.org/10.1016/J.JTCVS.2014.01.004
- Puskas JD, Gerdisch M, Nichols D, et al. Anticoagulation and Antiplatelet Strategies After On-X Mechanical Aortic Valve Replacement. J Am Coll Cardiol. 2018;71(24):2717-2726. https://doi.org/10.1016/J.JACC.2018.03.535
- Ussia GP, Scarabelli M, Mul M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2011;108(12):1772-1776. https://doi.org/10.1016/J.AMJCARD.2011.07.049
- Chakravarty T, Patel A, Kapadia S, et al. Anticoagulation After Surgical or Transcatheter Bioprosthetic Aortic Valve Replacement. J Am Coll Cardiol. 2019;74(9):1190-1200. https://doi.org/10.1016/J.JACC.2019.06.058
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N Engl J Med. 2015;373(21):2015-2024. https://doi.org/10.1056/NEJMOA1509233
- Jose J, Sulimov DS, El-Mawardy M, et al. Clinical Bioprosthetic Heart Valve Thrombosis After Transcatheter Aortic Valve Replacement: Incidence, Characteristics, and Treatment Outcomes. JACC Cardiovasc Interv. 2017;10(7):686-697. https://doi.org/10.1016/J.JCIN.2017.01.045
- Dangas GD, Tijssen JGP, Wöhrle J, et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N Engl J Med. 2020;382(2):120-129. https://doi.org/10.1056/NEJMOA1911425
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. https://doi.org/10.1056/NEJMOA0905561
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. https://doi.org/10.1056/NEJMOA1310907
- Summary of the article: Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med, 2011; 365: 1557–1559 | Szczerba | Kardiologia Polska (Polish Heart Journal). Accessed June 1, 2022. https://journals.viamedica.pl/kardiologia_polska/article/view/79117
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2011;365(11):981-992. https://doi.org/10.1056/NEJMOA1107039/SUPPL_FILE/NEJMOA1107039_DISCLOSURES.PDF
- Edmunds LH. Thrombotic and bleeding complications of prosthetic heart valves. Ann Thorac Surg. 1987;44(4):430-445. https://doi.org/10.1016/S0003-4975(10)63816-7
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165-3241. https://doi.org/10.1093/EURHEARTJ/EHY340
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/CHEST.11-2298
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336(21):1506-1511. https://doi.org/10.1056/NEJM199705223362107
- Tinker JH, Tarhan S. Discontinuing Anticoagulant Therapy in Surgical Patients With Cardiac Valve Prostheses: Observations in 180 Operations. JAMA. 1978;239(8):738-739. https://doi.org/10.1001/JAMA.1978.03280350062016
- Lankiewicz MW, Hays J, Friedman KD, Tinkoff G, Blatt PM. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost. 2006;4(5):967-970. https://doi.org/10.1111/J.1538-7836.2006.01815.X
- Renda G, Ricci F, Giugliano RP, de Caterina R. Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation and Valvular Heart Disease. J Am Coll Cardiol. 2017;69(11):1363-1371. https://doi.org/10.1016/J.JACC.2016.12.038
- Hammerstingl C, Tripp C, Schmidt H, von der Recke G, Omran H. Periprocedural Bridging Therapy with Low-Molecular-Weight Heparin in Chronically Anticoagulated Patients with Prosthetic Mechanical Heart Valves: Experience in 116 Patients from the Prospective BRAVE Registry. Published online 2007.
- Hjellström L, Labaf A. Prophylactic doses of low-molecular weight heparin as periprocedural bridging therapy in mechanical heart valve patients. Thromb Res. 2018;163:180-184. https://doi.org/10.1016/J.THROMRES.2017.09.023
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067. https://doi.org/10.1016/J.JACC.2017.09.1085
- Tsu L v., Dienes JE, Dager WE. Vitamin K dosing to reverse warfarin based on INR, route of administration, and home warfarin dose in the acute/critical care setting. Ann Pharmacother. 2012;46(12):1617-1626. https://doi.org/10.1345/APH.1R497
- Pernod G, Godiér A, Gozalo C, Tremey B, Sié P. French clinical practice guidelines on the management of patients on vitamin K antagonists in at-risk situations (overdose, risk of bleeding, and active bleeding). Thromb Res. 2010;126(3). https://doi.org/10.1016/J.THROMRES.2010.06.017
- Weibert RT, Le DT, Kayser SR, Rapaport SI. Correction of excessive anticoagulation with low-dose oral vitamin K1. Ann Intern Med. 1997;126(12):959-962. https://doi.org/10.7326/0003-4819-126-12-199706150-00005
- CV P, PA R, J E, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373(6). https://doi.org/10.1056/NEJMOA1502000
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015;373(25):2413-2424. https://doi.org/10.1056/NEJMOA1510991
- Connolly SJ, Milling TJ, Eikelboom JW, et al. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2016;375(12):1131-1141. https://doi.org/10.1056/NEJMOA1607887
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/NEJMOA1814051
- Dangas GD, Weitz JI, Giustino G, Makkar R, Mehran R. Prosthetic Heart Valve Thrombosis. J Am Coll Cardiol. 2016;68(24):2670-2689. https://doi.org/10.1016/J.JACC.2016.09.958
- Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389(10087):2383-2392. https://doi.org/10.1016/S0140-6736(17)30757-2
- Sondergaard L, de Backer O, Kofoed KF, et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur Heart J. 2017;38(28):2201-2207. https://doi.org/10.1093/EURHEARTJ/EHX369
- Montorsi P, de Bernardi F, Muratori M, Cavoretto D, Pepi M. Role of cine-fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol. 2000;85(1):58-64. https://doi.org/10.1016/S0002-9149(99)00607-4
- Muratori M, Montorsi P, Teruzzi G, et al. Feasibility and diagnostic accuracy of quantitative assessment of mechanical prostheses leaflet motion by transthoracic and transesophageal echocardiography in suspected prosthetic valve dysfunction. Am J Cardiol. 2006;97(1):94-100. https://doi.org/10.1016/J.AMJCARD.2005.07.112
- Cianciulli TF, Lax JA, Beck MA, et al. Cinefluoroscopic assessment of mechanical disc prostheses: its value as a complementary method to echocardiography. J Heart Valve Dis. 2005;14(5):664-673. Accessed June 1, 2022. https://europepmc.org/article/med/16245506
- Symersky P, Budde RPJ, de Mol BAJM, Prokop M. Comparison of multidetector-row computed tomography to echocardiography and fluoroscopy for evaluation of patients with mechanical prosthetic valve obstruction. Am J Cardiol. 2009;104(8):1128-1134. https://doi.org/10.1016/J.AMJCARD.2009.05.061
- Bapat V, Attia R, Redwood S, et al. Use of transcatheter heart valves for a valve-in-valve implantation in patients with degenerated aortic bioprosthesis: technical considerations and results. J Thorac Cardiovasc Surg. 2012;144(6):1372-1380. https://doi.org/10.1016/J.JTCVS.2012.07.104
- Gündüz S, Özkan M, Kalçik M, et al. Sixty-Four-Section Cardiac Computed Tomography in Mechanical Prosthetic Heart Valve Dysfunction: Thrombus or Pannus. Circ Cardiovasc Imaging. 2015;8(12). https://doi.org/10.1161/CIRCIMAGING.115.003246
- Suh YJ, Lee S, Im DJ, et al. Added value of cardiac computed tomography for evaluation of mechanical aortic valve: Emphasis on evaluation of pannus with surgical findings as standard reference. Int J Cardiol. 2016;214:454-460. https://doi.org/10.1016/J.IJCARD.2016.04.011
- Barbetseas J, Nagueh SF, Pitsavos C, Toutouzas PK, Quiñones MA, Zoghbi WA. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J Am Coll Cardiol. 1998;32(5):1410-1417. https://doi.org/10.1016/S0735-1097(98)00385-4
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the. J Am Soc Echocardiogr. 2009;22(9):975-1014. https://doi.org/10.1016/J.ECHO.2009.07.013
- Özkan M, Gündüz S, Biteker M, et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging. 2013;6(2):206-216. https://doi.org/10.1016/J.JCMG.2012.10.016
- Suchá D, Symersky P, Tanis W, et al. Multimodality Imaging Assessment of Prosthetic Heart Valves. Circ Cardiovasc Imaging. 2015;8(9). https://doi.org/10.1161/CIRCIMAGING.115.003703
- Cáceres-Lóriga FM, Pérez-López H, Morlans-Hernández K, et al. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J Thromb Thrombolysis. 2006;21(2):185-190. https://doi.org/10.1007/S11239-006-4969-Y
- Özkan M, Çakal B, Karakoyun S, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation. 2013;128(5):532-540. https://doi.org/10.1161/CIRCULATIONAHA.113.001145
- Karthikeyan G, Senguttuvan NB, Joseph J, Devasenapathy N, Bahl VK, Airan B. Urgent surgery compared with fibrinolytic therapy for the treatment of left-sided prosthetic heart valve thrombosis: a systematic review and meta-analysis of observational studies. Eur Heart J. 2013;34(21):1557-1566. https://doi.org/10.1093/EURHEARTJ/EHS486
- Keuleers S, Herijgers P, Herregods MC, et al. Comparison of thrombolysis versus surgery as a first line therapy for prosthetic heart valve thrombosis. Am J Cardiol. 2011;107(2):275-279. https://doi.org/10.1016/J.AMJCARD.2010.09.013
- Nagy A, Nagy A, Dénes M, Lengyel M. Predictors of the Outcome of Thrombolytic Therapy in Prosthetic Mitral Valve Thrombosis: A Study of 62 Events Emiratis vs South Asian Young Patients With Acute Coronary Syndromes: Risk Factor Profiles, Presentations and In-Hospital Outcomes View project Predictors of the Outcome of Thrombolytic Therapy in Prosthetic Mitral Valve Thrombosis: A Study of 62 Events. Published online 2014. Accessed June 1, 2022. https://www.researchgate.net/publication/26322239
- Roudaut R, Lafitte S, Roudaut MF, et al. Management of prosthetic heart valve obstruction: fibrinolysis versus surgery. Early results and long-term follow-up in a single-centre study of 263 cases. Arch Cardiovasc Dis. 2009;102(4):269-277. https://doi.org/10.1016/J.ACVD.2009.01.007
- Tong AT, Roudaut R, Ozkan M, et al. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol. 2004;43(1):77-84. https://doi.org/10.1016/J.JACC.2003.08.028
- Bade AS, Shaikh SSA, Khemani H, Singh G, Bansal NO. Thrombolysis Is an Effective and Safe Therapy in Stuck Mitral Valves With Delayed Presentation as Well as Hemodynamically Unstable Patients: A Single Centre Study. Cardiol Res. 2018;9(3):161-164. https://doi.org/10.14740/CR708W
- Pragt H, van Melle JP, Javadikasgari H, et al. Mechanical valves in the pulmonary position: An international retrospective analysis. J Thorac Cardiovasc Surg. 2017;154(4):1371-1378.e1. https://doi.org/10.1016/J.JTCVS.2017.04.072
- Taherkhani M, Hashemi SR, Hekmat M, Safi M, Taherkhani A, Movahed MR. Thrombolytic Therapy for Right-Sided Mechanical Pulmonic and Tricuspid Valves: The Largest Survival Analysis to Date. Tex Heart Inst J. 2015;42(6):543-547. https://doi.org/10.14503/THIJ-14-4659
- Özkan M, Gündüz S, Gürsoy OM, et al. Ultraslow thrombolytic therapy: A novel strategy in the management of PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE: The Ultra-slow PROMETEE trial. Am Heart J. 2015;170(2):409-418.e1. https://doi.org/10.1016/J.AHJ.2015.04.025
- Puri R, Auffret V, Rodés-Cabau J. Bioprosthetic Valve Thrombosis. J Am Coll Cardiol. 2017;69(17):2193-2211. https://doi.org/10.1016/J.JACC.2017.02.051
- Puvimanasinghe JPA, Steyerberg EW, Takkenberg JJM, et al. Prognosis after aortic valve replacement with a bioprosthesis: predictions based on meta-analysis and microsimulation. Circulation. 2001;103(11):1535-1541. https://doi.org/10.1161/01.CIR.103.11.1535
- Jander N, Kienzle RP, Kayser G, Neumann FJ, Gohlke-Baerwolf C, Minners J. Usefulness of phenprocoumon for the treatment of obstructing thrombus in bioprostheses in the aortic valve position. Am J Cardiol. 2012;109(2):257-262. https://doi.org/10.1016/J.AMJCARD.2011.08.038
- Butnaru A, Shaheen J, Tzivoni D, Tauber R, Bitran D, Silberman S. Diagnosis and treatment of early bioprosthetic malfunction in the mitral valve position due to thrombus formation. Am J Cardiol. 2013;112(9):1439-1444. https://doi.org/10.1016/J.AMJCARD.2013.06.014
- Pislaru S v., Hussain I, Pellikka PA, et al. Misconceptions, diagnostic challenges and treatment opportunities in bioprosthetic valve thrombosis: lessons from a case series. Eur J Cardiothorac Surg. 2015;47(4):725-732. https://doi.org/10.1093/EJCTS/EZU201
- de Marchena E, Mesa J, Pomenti S, et al. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8(5):728-739. https://doi.org/10.1016/J.JCIN.2015.03.005
- Petrescu I, Egbe AC, Ionescu F, et al. Long-Term Outcomes of Anticoagulation for Bioprosthetic Valve Thrombosis. J Am Coll Cardiol. 2020;75(8):857-866. https://doi.org/10.1016/J.JACC.2019.12.037
- Sellers SL, Turner CT, Sathananthan J, et al. Transcatheter Aortic Heart Valves: Histological Analysis Providing Insight to Leaflet Thickening and Structural Valve Degeneration. JACC Cardiovasc Imaging. 2019;12(1):135-145. https://doi.org/10.1016/J.JCMG.2018.06.028
- Latib A, Naganuma T, Abdel-Wahab M, et al. Treatment and clinical outcomes of transcatheter heart valve thrombosis. Circ Cardiovasc Interv. 2015;8(4). https://doi.org/10.1161/CIRCINTERVENTIONS.114.001779
- Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(6):589-590. https://doi.org/10.1093/EHJCI/JEW025
- Leontyev S, Borger MA, Davierwala P, et al. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg. 2011;91(4):1120-1126. https://doi.org/10.1016/J.ATHORACSUR.2010.12.053
- Kaneko T, Vassileva CM, Englum B, et al. Contemporary Outcomes of Repeat Aortic Valve Replacement: A Benchmark for Transcatheter Valve-in-Valve Procedures. Ann Thorac Surg. 2015;100(4):1298-1304. https://doi.org/10.1016/J.ATHORACSUR.2015.04.062
- Jaussaud N, Gariboldi V, Grisoli D, et al. Risk of reoperation for mitral bioprosthesis dysfunction. J Heart Valve Dis. 2012;21(1):56-60. Accessed June 4, 2022. https://europepmc.org/article/med/22474743
- Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312(2):162-170. https://doi.org/10.1001/JAMA.2014.7246
- Ye J, Cheung A, Yamashita M, et al. Transcatheter Aortic and Mitral Valve-in-Valve Implantation for Failed Surgical Bioprosthetic Valves: An 8-Year Single-Center Experience. JACC Cardiovasc Interv. 2015;8(13):1735-1744. https://doi.org/10.1016/J.JCIN.2015.08.012
- Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. Journal of the American Society of Echocardiography. 2019;32(4):431-475. https://doi.org/10.1016/J.ECHO.2019.01.003
- Hascoet S, Smolka G, Bagate F, et al. Multimodality imaging guidance for percutaneous paravalvular leak closure: Insights from the multi-centre FFPP register. Arch Cardiovasc Dis. 2018;111(6-7):421-431. https://doi.org/10.1016/J.ACVD.2018.05.001
- García-Fernández MA, Cortés M, García-Robles JA, Gomez de Diego JJ, Perez-David E, García E. Utility of real-time three-dimensional transesophageal echocardiography in evaluating the success of percutaneous transcatheter closure of mitral paravalvular leaks. J Am Soc Echocardiogr. 2010;23(1):26-32. https://doi.org/10.1016/J.ECHO.2009.09.028
- Nombela-Franco L, Ribeiro HB, Urena M, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol. 2014;63(24):2643-2658. https://doi.org/10.1016/J.JACC.2014.02.573
- Ruiz CE, Hahn RT, Berrebi A, et al. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis: An Expert Statement. J Am Coll Cardiol. 2017;69(16):2067-2087. https://doi.org/10.1016/J.JACC.2017.02.038
- Akins CW, Bitondo JM, Hilgenberg AD, Vlahakes GJ, Madsen JC, MacGillivray TE. Early and late results of the surgical correction of cardiac prosthetic paravalvular leaks. J Heart Valve Dis. 2005;14(6):792-799; discussion 799. Accessed June 4, 2022. https://europepmc.org/article/med/16359061
- Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Percutaneous repair of paravalvular prosthetic regurgitation: acute and 30-day outcomes in 115 patients. Circ Cardiovasc Interv. 2011;4(4):314-321. https://doi.org/10.1161/CIRCINTERVENTIONS.110.960955
- Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgitation. J Am Coll Cardiol. 2011;58(21):2218-2224. https://doi.org/10.1016/J.JACC.2011.07.041
- Alkhouli M, Rihal CS, Zack CJ, et al. Transcatheter and Surgical Management of Mitral Paravalvular Leak: Long-Term Outcomes. JACC Cardiovasc Interv. 2017;10(19):1946-1956. https://doi.org/10.1016/J.JCIN.2017.07.046
- Alkhouli M, Zack CJ, Sarraf M, et al. Successful Percutaneous Mitral Paravalvular Leak Closure Is Associated With Improved Midterm Survival. Circ Cardiovasc Interv. 2017;10(12). https://doi.org/10.1161/CIRCINTERVENTIONS.117.005730
- Ruiz CE, Jelnin V, Kronzon I, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol. 2011;58(21):2210-2217. https://doi.org/10.1016/J.JACC.2011.03.074
- Phan K, Zhao DF, Wang N, Huo YR, Eusanio M di, Yan TD. Transcatheter valve-in-valve implantation versus reoperative conventional aortic valve replacement: a systematic review. J Thorac Dis. 2016;8(1):E83-E93. https://doi.org/10.3978/J.ISSN.2072-1439.2016.01.44
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121(16):1848-1857. https://doi.org/10.1161/CIRCULATIONAHA.109.924613
- Shah S, Alashi A, Pettersson GB, et al. Characteristics and longer-term outcomes of paravalvular leak after aortic and mitral valve surgery. J Thorac Cardiovasc Surg. 2019;157(5):1785-1792.e1. https://doi.org/10.1016/J.JTCVS.2018.08.096
- Bouhout I, Mazine A, Ghoneim A, et al. Long-term results after surgical treatment of paravalvular leak in the aortic and mitral position. J Thorac Cardiovasc Surg. 2016;151(5):1260-1266.e1. https://doi.org/10.1016/J.JTCVS.2015.11.046
- Karchmer AW, Chu VH, Otto CM. Prosthetic valve endocarditis: Epidemiology, clinical manifestations, and diagnosis.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633-638. https://doi.org/10.1086/313753
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol. 2017;69(3):325-344. https://doi.org/10.1016/J.JACC.2016.10.066
- Mgbojikwe N, Jones SR, Leucker TM, Brotman DJ. Infective endocarditis: Beyond the usual tests. Cleve Clin J Med. 2019;86(8):559-567. https://doi.org/10.3949/CCJM.86A.18120
- Mahmood M, Kendi AT, Ajmal S, et al. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J Nucl Cardiol. 2019;26(3):922-935. https://doi.org/10.1007/S12350-017-1092-8
- Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/JAMAINTERNMED.2014.2556
- Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. New England Journal of Medicine. 2019;380(5):415-424.
- Prendergast BD, Tornos P. Surgery for infective endocarditis: Who and when? Circulation. 2010;121(9):1141-1152. https://doi.org/10.1161/CIRCULATIONAHA.108.773598
- Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013;173(16):1495-1504.
- Fowler VG, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293(24):3012-3021.
- Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J. 2007;28(2):196-203.
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis–Prospective Cohort Study. Arch Intern Med. 2009;169(5):463-473.
- Spiliopoulos K, Haschemi A, Fink G, Kemkes BM. Infective endocarditis complicated by paravalvular abscess: A surgical challenge. An 11-year single center experience. Heart Surgery Forum. 2010;13(2). https://doi.org/10.1532/HSF98.20081141
- Head SJ, Mokhles MM, Osnabrugge RLJ, Bogers AJJC, Kappetein AP. Surgery in current therapy for infective endocarditis. Vasc Health Risk Manag. 2011;7:255.
- Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458-477. https://doi.org/10.1161/CIRCULATIONAHA.109.192665
- Lin AY, Saul T, Aldaas OM, et al. Early versus delayed lead extraction in patients with infected cardiovascular implantable electronic devices. JACC Clin Electrophysiol. 2021;7(6):755-763.
- Ghoreishi M, Foster N, Pasrija C, et al. Early operation in patients with mitral valve infective endocarditis and acute stroke is safe. Ann Thorac Surg. 2018;105(1):69-75.
- Orwat S, Diller GP, van Hagen IM, et al. Risk of Pregnancy in Moderate and Severe Aortic Stenosis: From the Multinational ROPAC Registry. J Am Coll Cardiol. 2016;68(16):1727-1737. https://doi.org/10.1016/J.JACC.2016.07.750
- Tzemos N, Silversides CK, Colman JM, et al. Late cardiac outcomes after pregnancy in women with congenital aortic stenosis. Am Heart J. 2009;157(3):474-480. https://doi.org/10.1016/J.AHJ.2008.10.020
- Arias F, J. Pineda. Aortic stenosis and pregnancy.
- Silversides CK, Colman JM, Sermer M, Farine D, Siu SC. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol. 2003;91(11):1386-1389. https://doi.org/10.1016/S0002-9149(03)00340-0
- Yap SC, Drenthen W, Pieper PG, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol. 2008;126(2):240-246. https://doi.org/10.1016/J.IJCARD.2007.03.134
- Sugishita Y, Ito I, Kubo T. Pregnancy in Cardiac Patients: Possible Influence of Volume Overload by Pregnancy on Pulmonary Circulation : PANEL DISCUSSION ON PUMP FAILURE OF THE HEART WITH COMPLICATIONS : 49th Annual Scientific Session of the Japanese Circulation Society. Jpn Circ J. 1986;50(4):376-383. https://doi.org/10.1253/JCJ.50.376
- de Santo LS, Romano G, della Corte A, et al. Mechanical Aortic Valve Replacement in Young Women Planning on Pregnancy: Maternal and Fetal Outcomes Under Low Oral Anticoagulation, a Pilot Observational Study on a Comprehensive Pre-Operative Counseling Protocol. J Am Coll Cardiol. 2012;59(12):1110-1115. https://doi.org/10.1016/J.JACC.2011.10.899
- Leśniak-Sobelga A, Tracz W, Kostkiewicz M, Podolec P, Pasowicz M. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases—maternal and fetal outcome. Int J Cardiol. 2004;94(1):15-23. https://doi.org/10.1016/J.IJCARD.2003.03.017
- Hameed A, Karaalp IS, Tummala PP, et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001;37(3):893-899. https://doi.org/10.1016/S0735-1097(00)01198-0
- Orwat S, Diller GP, van Hagen IM, et al. Risk of Pregnancy in Moderate and Severe Aortic Stenosis: From the Multinational ROPAC Registry. J Am Coll Cardiol. 2016;68(16):1727-1737. https://doi.org/10.1016/J.JACC.2016.07.750
- Vinayakumar D, Vinod G v., Madhavan S, Krishnan MN. Maternal and fetal outcomes in pregnant women undergoing balloon mitral valvotomy for rheumatic mitral stenosis. Indian Heart J. 2016;68(6):780-782. https://doi.org/10.1016/J.IHJ.2016.04.017
- Gulraze A, Kurdi W, Niaz FA, Fawzy ME. Mitral balloon valvuloplasty during pregnancy:The long term up to 17 years obstetric outcome and childhood development. Pak J Med Sci. 2014;30(1):86. https://doi.org/10.12669/PJMS.301.4305
- Salomé N, Dias CC, Ribeiro J, Gonçalves M, Fonseca C, Ribeiro VG. Balloon mitral valvuloplasty during pregnancy–our experience. Rev Port Cardiol. 2002;21(12):1437-1444. Accessed August 29, 2022. https://europepmc.org/article/med/12621917
- Weiss BM, von Segesser LK, Alon E, Seifert B, Turina MI. Outcome of cardiovascular surgery and pregnancy: A systematic review of the period 1984-1996. Am J Obstet Gynecol. 1998;179(6):1643-1653. https://doi.org/10.1016/S0002-9378(98)70039-0
- Becker RM. Intracardiac Surgery in Pregnant Women. Ann Thorac Surg. 1983;36(4):453-458. https://doi.org/10.1016/S0003-4975(10)60486-9
- Parry AJ, Westaby S. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 1996;61(6):1865-1869. https://doi.org/10.1016/0003-4975(96)00150-6
- Samiei N, Amirsardari M, Rezaei Y, et al. Echocardiographic Evaluation of Hemodynamic Changes in Left-Sided Heart Valves in Pregnant Women With Valvular Heart Disease. Am J Cardiol. 2016;118(7):1046-1052. https://doi.org/10.1016/J.AMJCARD.2016.07.005
- D’Souza R, Ostro J, Shah PS, et al. Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J. 2017;38(19):1509-1516. https://doi.org/10.1093/EURHEARTJ/EHX032
- Özkan M, Çakal B, Karakoyun S, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation. 2013;128(5):532-540. https://doi.org/10.1161/CIRCULATIONAHA.113.001145
- Tong AT, Roudaut R, Ozkan M, et al. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol. 2004;43(1):77-84. https://doi.org/10.1016/J.JACC.2003.08.028
- Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(6):589-590. https://doi.org/10.1093/EHJCI/JEW025
- Massel DR, Little SH. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2013;2013(7). https://doi.org/10.1002/14651858.CD003464.PUB2
- Garrett AD. Dabigatran vs. Warfarin in patients with mechanical heart valves. Drug Topics. 2013;369(DEC):1206-1220. https://doi.org/10.1056/NEJMOA1300615/SUPPL_FILE/NEJMOA1300615_DISCLOSURES.PDF
- Bajaj A, Pancholy S, Sethi A, Rathor P. Safety and feasibility of PCI in patients undergoing TAVR: A systematic review and meta-analysis. Heart Lung. 2017;46(2):92-99. https://doi.org/10.1016/J.HRTLNG.2016.12.003
- Chakravarty T, Sharma R, Abramowitz Y, et al. Outcomes in Patients With Transcatheter Aortic Valve Replacement and Left Main Stenting: The TAVR-LM Registry. J Am Coll Cardiol. 2016;67(8):951-960. https://doi.org/10.1016/J.JACC.2015.10.103
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629-638. https://doi.org/10.1016/S0140-6736(13)60141-5
- Thalji NM, Suri RM, Daly RC, et al. The prognostic impact of concomitant coronary artery bypass grafting during aortic valve surgery: implications for revascularization in the transcatheter era. J Thorac Cardiovasc Surg. 2015;149(2):451-460.e2. https://doi.org/10.1016/J.JTCVS.2014.08.073
- Faroux L, Guimaraes L, Wintzer-Wehekind J, et al. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74(3):362-372. https://doi.org/10.1016/J.JACC.2019.06.012
- Goel SS, Ige M, Tuzcu EM, et al. Severe aortic stenosis and coronary artery disease–implications for management in the transcatheter aortic valve replacement era: a comprehensive review. J Am Coll Cardiol. 2013;62(1):1-10. https://doi.org/10.1016/J.JACC.2013.01.096
- Sankaramangalam K, Banerjee K, Kandregula K, et al. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J Am Heart Assoc. 2017;6(10). https://doi.org/10.1161/JAHA.117.006092
- Millan-Iturbe O, Sawaya FJ, Lønborg J, et al. Coronary artery disease, revascularization, and clinical outcomes in transcatheter aortic valve replacement: Real-world results from the East Denmark Heart Registry. Catheter Cardiovasc Interv. 2018;92(4):818-826. https://doi.org/10.1002/CCD.27440
- Witberg G, Regev E, Chen S, et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10(14):1428-1435. https://doi.org/10.1016/J.JCIN.2017.04.035
- Chieffo A, Giustino G, Spagnolo P, et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2015;8(7). https://doi.org/10.1161/CIRCINTERVENTIONS.114.002025
- Rossi A, de Cecco CN, Kennon SRO, et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2017;11(5):338-346. https://doi.org/10.1016/J.JCCT.2017.06.001
- van den Boogert TPW, Vendrik J, Claessen BEPM, et al. CTCA for detection of significant coronary artery disease in routine TAVI work-up : A systematic review and meta-analysis. Neth Heart J. 2018;26(12):591-599. https://doi.org/10.1007/S12471-018-1149-6
- Pesarini G, Scarsini R, Zivelonghi C, et al. Functional Assessment of Coronary Artery Disease in Patients Undergoing Transcatheter Aortic Valve Implantation: Influence of Pressure Overload on the Evaluation of Lesions Severity. Circ Cardiovasc Interv. 2016;9(11). https://doi.org/10.1161/CIRCINTERVENTIONS.116.004088
- Ahmad Y, Götberg M, Cook C, et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc Interv. 2018;11(20):2019-2031. https://doi.org/10.1016/J.JCIN.2018.07.019
- Yamanaka F, Shishido K, Ochiai T, et al. Instantaneous Wave-Free Ratio for the Assessment of Intermediate Coronary Artery Stenosis in Patients With Severe Aortic Valve Stenosis: Comparison With Myocardial Perfusion Scintigraphy. JACC Cardiovasc Interv. 2018;11(20):2032-2040. https://doi.org/10.1016/J.JCIN.2018.07.027
- Graboys TB, Cohn PF. The prevalence of angina pectoris and abnormal coronary arteriograms in severe aortic valvular disease. Am Heart J. 1977;93(6):683-686. https://doi.org/10.1016/S0002-8703(77)80062-8
- Ramsdale DR, Bennett DH, Bray CL, Ward C, Beton DC, Faragher EB. Angina, coronary risk factors and coronary artery disease in patients with valvular disease. A prospective study. Eur Heart J. 1984;5(9):716-726. https://doi.org/10.1093/OXFORDJOURNALS.EURHEARTJ.A061732
- Dangas G, Khan S, Curry BH, Kini AS, Sharma SK. Angina pectoris in severe aortic stenosis. Cardiology. 1999;92(1):1-3. https://doi.org/10.1159/000006938
- Basta LL, Raines D, Najjar S, Kioschos JM. Clinical, haemodynamic, and coronary angiographic correlates of angina pectoris in patients with severe aortic valve disease. Br Heart J. 1975;37(2):150-157. https://doi.org/10.1136/HRT.37.2.150
- Thalji NM, Suri RM, Daly RC, et al. Assessment of coronary artery disease risk in 5463 patients undergoing cardiac surgery: when is preoperative coronary angiography necessary? J Thorac Cardiovasc Surg. 2013;146(5). https://doi.org/10.1016/J.JTCVS.2013.06.046
- Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38(1):46-51. https://doi.org/10.1016/0002-9149(76)90061-8
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350-1358. https://doi.org/10.1056/NEJM197906143002402
- Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95(3):252-258. https://doi.org/10.1136/HRT.2008.149088
- Gahl K, Sutton R, Pearson M, Caspari P, Lairet A, McDonald L. Mitral regurgitation in coronary heart disease. Br Heart J. 1977;39(1):13-18. https://doi.org/10.1136/HRT.39.1.13
- Enriquez-Sarano M, Klodas E, Garratt KN, Bailey KR, Tajik AJ, Holmes DR. Secular trends in coronary atherosclerosis–analysis in patients with valvular regurgitation. N Engl J Med. 1996;335(5):316-322. https://doi.org/10.1056/NEJM199608013350504
- Breisblatt WM, Cerqueira M, Francis CK, Plankey M, Zaret BL, Berger HJ. Left ventricular function in ischemic mitral regurgitation–a precatheterization assessment. Am Heart J. 1988;115(1 Pt 1):77-82. https://doi.org/10.1016/0002-8703(88)90520-0
- Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699. https://doi.org/10.1016/J.JACC.2009.11.013
- Opolski MP, Kim WK, Liebetrau C, et al. Diagnostic accuracy of computed tomography angiography for the detection of coronary artery disease in patients referred for transcatheter aortic valve implantation. Clin Res Cardiol. 2015;104(6):471-480. https://doi.org/10.1007/S00392-014-0806-Z
- Andreini D, Pontone G, Mushtaq S, et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am Heart J. 2014;168(3):332-339. https://doi.org/10.1016/J.AHJ.2014.04.022
- Chieffo A, Giustino G, Spagnolo P, et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2015;8(7). https://doi.org/10.1161/CIRCINTERVENTIONS.114.002025
- Matsumoto S, Yamada Y, Hashimoto M, et al. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur Radiol. 2017;27(5):1963-1970. https://doi.org/10.1007/S00330-016-4547-4
- Rossi A, de Cecco CN, Kennon SRO, et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2017;11(5):338-346. https://doi.org/10.1016/J.JCCT.2017.06.001
- Byrne JG, Leacche M, Vaughan DE, Zhao DX. Hybrid cardiovascular procedures. JACC Cardiovasc Interv. 2008;1(5):459-468. https://doi.org/10.1016/J.JCIN.2008.07.002
- Hamdan A, Wellnhofer E, Konen E, et al. Coronary CT angiography for the detection of coronary artery stenosis in patients referred for transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2015;9(1):31-41. https://doi.org/10.1016/J.JCCT.2014.11.008
- Pontone G, Andreini D, Bartorelli AL, et al. Feasibility and accuracy of a comprehensive multidetector computed tomography acquisition for patients referred for balloon-expandable transcatheter aortic valve implantation. Am Heart J. 2011;161(6):1106-1113. https://doi.org/10.1016/J.AHJ.2011.03.003
- Lund O, Nielsen TT, Pilegaard HK, Magnussen K, Knudsen MA. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 1990;100(3):327-337. https://doi.org/10.1016/S0022-5223(19)35524-2
- Beach JM, Mihaljevic T, Svensson LG, et al. Coronary artery disease and outcomes of aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2013;61(8):837-848. https://doi.org/10.1016/J.JACC.2012.10.049
- Ad N, Henry L, Hunt S, Holmes SD. Do we increase the operative risk by adding the Cox Maze III procedure to aortic valve replacement and coronary artery bypass surgery? J Thorac Cardiovasc Surg. 2012;143(4):936-944. https://doi.org/10.1016/J.JTCVS.2011.12.018
- Badhwar V, Rankin JS, Damiano RJ, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg. 2017;103(1):329-341. https://doi.org/10.1016/J.ATHORACSUR.2016.10.076
- Ad N, Holmes SD, Pritchard G, Shuman DJ. Association of operative risk with the outcome of concomitant Cox Maze procedure: a comparison of results across risk groups. J Thorac Cardiovasc Surg. 2014;148(6):3027-3033. https://doi.org/10.1016/J.JTCVS.2014.05.039
- Ad N, Holmes SD, Rongione AJ, et al. The long-term safety and efficacy of concomitant Cox maze procedures for atrial fibrillation in patients without mitral valve disease. J Thorac Cardiovasc Surg. 2019;157(4):1505-1514. https://doi.org/10.1016/J.JTCVS.2018.09.131
- Gillinov AM, Bakaeen F, McCarthy PM, et al. Surgery for paroxysmal atrial fibrillation in the setting of mitral valve disease: a role for pulmonary vein isolation? Ann Thorac Surg. 2006;81(1):19-28. https://doi.org/10.1016/J.ATHORACSUR.2005.04.060
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372(15):1399-1409. https://doi.org/10.1056/NEJMOA1500528
- Abreu Filho CAC, Lisboa LAF, Dallan LAO, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation. 2005;112(9 Suppl). https://doi.org/10.1161/CIRCULATIONAHA.104.526301
- Akpinar B, Guden M, Sagbas E, et al. Combined radiofrequency modified maze and mitral valve procedure through a port access approach: early and mid-term results. Eur J Cardiothorac Surg. 2003;24(2):223-230. https://doi.org/10.1016/S1010-7940(03)00258-6
- Chua YL, Schaff H v., Orszulak TA, Morris JJ. Outcome of mitral valve repair in patients with preoperative atrial fibrillation: Should the maze procedure be combined with mitral valvuloplasty? J Thorac Cardiovasc Surg. 1994;107(2):408-415. https://doi.org/10.1016/S0022-5223(12)70085-5
- Deneke T, Khargi K, Grewe PH, et al. Efficacy of an additional MAZE procedure using cooled-tip radiofrequency ablation in patients with chronic atrial fibrillation and mitral valve disease. A randomized, prospective trial. Eur Heart J. 2002;23(7):558-566. https://doi.org/10.1053/EUHJ.2001.2841
- Jessurun ER, van Hemel NM, Defauw JJ, de La Rivière AB. A randomized study of combining maze surgery for atrial fibrillation with mitral valve surgery. Journal of Cardiovascular Surgery. 2003;44(1):9.
- Ad N, Holmes SD, Lamont D, Shuman DJ. Left-Sided Surgical Ablation for Patients With Atrial Fibrillation Who Are Undergoing Concomitant Cardiac Surgical Procedures. Ann Thorac Surg. 2017;103(1):58-65. https://doi.org/10.1016/J.ATHORACSUR.2016.05.093
- Huffman MD, Karmali KN, Berendsen MA, et al. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database Syst Rev. 2016;2016(8). https://doi.org/10.1002/14651858.CD011814.PUB2
- Huffman MD, Malaisrie SC, Karmali KN. Concomitant Atrial Fibrillation Surgery for People Undergoing Cardiac Surgery. JAMA Cardiol. 2017;2(3):334-335. https://doi.org/10.1001/JAMACARDIO.2016.5404
- Friedman DJ, Piccini JP, Wang T, et al. Association Between Left Atrial Appendage Occlusion and Readmission for Thromboembolism Among Patients With Atrial Fibrillation Undergoing Concomitant Cardiac Surgery. JAMA. 2018;319(4):365-374. https://doi.org/10.1001/JAMA.2017.20125
- Yao X, Gersh BJ, Holmes DR, et al. Association of Surgical Left Atrial Appendage Occlusion With Subsequent Stroke and Mortality Among Patients Undergoing Cardiac Surgery. JAMA. 2018;319(20):2116-2126. https://doi.org/10.1001/JAMA.2018.6024
- Johnsrud DO, Melduni RM, Lahr B, Yao X, Greason KL, Noseworthy PA. Evaluation of anticoagulation use and subsequent stroke in patients with atrial fibrillation after empiric surgical left atrial appendage closure: A retrospective case-control study. Clin Cardiol. 2018;41(12):1578-1582. https://doi.org/10.1002/CLC.23066
- Abrich VA, Narichania AD, Love WT, Lanza LA, Shen WK, Sorajja D. Left atrial appendage exclusion during mitral valve surgery and stroke in atrial fibrillation. J Interv Card Electrophysiol. 2018;53(3):285-292. https://doi.org/10.1007/S10840-018-0458-4
- García-Fernández MÁ, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003;42(7):1253-1258. https://doi.org/10.1016/S0735-1097(03)00954-9
- Noseworthy PA, Yao X, Deshmukh AJ, et al. Patterns of Anticoagulation Use and Cardioembolic Risk After Catheter Ablation for Atrial Fibrillation. J Am Heart Assoc. 2015;4(11). https://doi.org/10.1161/JAHA.115.002597
- Eitel C, Koch J, Sommer P, et al. Novel oral anticoagulants in a real-world cohort of patients undergoing catheter ablation of atrial fibrillation. Europace. 2013;15(11):1587-1593. https://doi.org/10.1093/EUROPACE/EUT128
- Melduni RM, Schaff H v., Lee HC, et al. Impact of Left Atrial Appendage Closure During Cardiac Surgery on the Occurrence of Early Postoperative Atrial Fibrillation, Stroke, and Mortality: A Propensity Score-Matched Analysis of 10 633 Patients. Circulation. 2017;135(4):366-378. https://doi.org/10.1161/CIRCULATIONAHA.116.021952
- Rankin JS, Grau-Sepulveda M v., Ad N, et al. Associations Between Surgical Ablation and Operative Mortality After Mitral Valve Procedures. Ann Thorac Surg. 2018;105(6):1790-1796. https://doi.org/10.1016/J.ATHORACSUR.2017.12.035