Trưởng ban: PGS.TS. PHẠM NGUYỄN VINH
Tham gia biên soạn: PGS.TS. HỒ HUỲNH QUANG TRÍ,
TS.BS. TRẦN VŨ MINH THƯ, BS CKII. LÊ THỊ ĐẸP,
BS CKII. TRẦN THỊ TUYẾT LAN, ThS.BS. HUỲNH THANH KIỀU,
ThS.BS. PHẠM ĐỖ ANH THƯ, BS CKI. PHẠM THỤC MINH THỦY
Biên tập: LƯƠNG BÍCH NHUNG, TRẦN THỊ THANH NGA
(…)
12. Hẹp van hai lá
Nguyên nhân gây hẹp van hai lá chủ yếu là thấp tim và thoái hóa. Thấp tim là nguyên nhân gây hẹp van hai lá hàng đầu trên toàn thế giới. Mặc dù tỷ lệ mắc thấp tim đã giảm đáng kể ở các nước phát triển nhưng vẫn còn là một vấn đề nhức nhối ở các nước đang phát triển, đặc biệt ở người trẻ. Hẹp hai lá do thoái hóa liên quan đến vôi hóa vòng van hai lá là một cơ chế riêng và tỷ lệ của bệnh sẽ tăng một cách có ý nghĩa theo tuổi. Hẹp hai lá do hai nguyên nhân này đều gặp nhiều hơn ở nữ giới. Ngoài ra, trong một số ít các trường hợp, hẹp hai lá do lá van cứng mà không có dính các mẹp van có thể do xạ trị, bệnh tim carcinoid hoặc các bệnh chuyển hóa di truyền.
12.1. Các giai đoạn của hẹp van hai lá
Các giai đoạn của hẹp van hai lá được xác định dựa trên triệu chứng, giải phẫu van hai lá, huyết động van hai lá và hậu quả của tắc nghẽn van hai lá lên nhĩ trái và tuần hoàn phổi (Bảng 17). Bệnh van hai lá do thấp là nguyên nhân chính gây hẹp hai lá với đặc điểm giải phẫu phản ánh quá trình tiến triển của bệnh. Mức độ ảnh hưởng lên huyết động được thể hiện qua diện tích van đo bằng phương pháp vẽ viền trực tiếp trên hình ảnh siêu âm tim 2D hoặc 3D hoặc tính bằng thời gian bán giảm áp lực (PHT). Định nghĩa hẹp hai lá khít dựa trên mức độ nặng của triệu chứng cũng như mức độ hẹp van mà can thiệp có thể giúp cải thiện triệu chứng. Vì vậy diện tích lỗ van hai lá ≤ 1,5 cm2 được coi là hẹp hai lá khít, thường tương ứng với chênh áp trung bình qua van từ trên 5 mmHg đến 10 mmHg ở tần số tim bình thường. Tuy nhiên, chênh áp trung bình qua van hai lá phụ thuộc tốc độ dòng chảy, thời gian đổ đầy tâm trương và tần số tim. Thời gian bán giảm áp lực cũng có những hạn chế nhất định và phục thuộc vào khả năng giãn (compliance) của nhĩ trái và thất trái cũng như mức độ hẹp hai lá. Có thể sử dụng các phương pháp tính diện tích lỗ van hai lá khác như sử dụng phương trình liên tục hoặc công thức Gorlin nếu kết quả đo không thống nhất. Điều này thường xảy ra trong hẹp hai lá do thấp.
12.2. Hẹp van hai lá hậu thấp
12.2.1.Đánh giá
Hẹp khít van hai lá được xác định khi diện tích van hai lá ≤ 1,5 cm2. Dính các mép van kèm theo dày lá van sau là cơ chế quan trọng nhất gây hẹp. Siêu âm tim là phương tiện đầu tay để chẩn đoán, đánh giá mức độ hẹp và các ảnh hưởng huyết động do hẹp van hai lá. Trong khi đo diện tích lỗ van bằng cách vẽ viền trên siêu âm tim 2D là phương pháp để đánh giá mức độ hẹp của van, chênh áp trung bình qua van và áp lực động mạch phổi phản ánh những hậu quả về huyết động và có vai trò tiên lượng bệnh. Đo diện tích van bằng siêu âm tim 3D qua thành ngực cũng có giá trị hỗ trợ chẩn đoán. Siêu âm tim qua thành ngực thường cung cấp thông tin đủ để quản lý bệnh một cách thường quy. Các thang điểm đã được xây dựng nhằm đánh giá mức độ phù hợp để can thiệp nong van hai lá qua da. Nên tiến hành siêu âm thực quản để loại
Bảng 17. Các giai đoạn trong hẹp van hai lá theo khuyến cáo của ACC/AHA năm 2020 (1)
| Định nghĩa | Giải phẫu van | Huyết động van | Hậu quả huyết động | Triệu chứng |
| · Giai đoạn A | ||||
| Có nguy cơ hẹp hai lá | · Van hai lá mở dạng vòm trong thì tâm trương | · Dòng chảy qua van bình thường | Không | Không |
| · Giai đoạn B | ||||
| Hẹp hai lá tiến triển | · Tổn thương van do thấp với dính mép van và các lá van mở dạng vòm trong thì tâm trương.
· Diện tích lỗ van trên 1,5 cm2 đo viền trực tiếp. |
· Tăng tốc độ dòng chảy qua van.
· Diện tích lỗ van trên 1,5 cm2. · Thời gian bán giảm áp lực tâm trương dưới 150 ms. |
· Nhĩ trái giãn nhẹ tới vừa
· Áp lực động mạch phổi lúc nghỉ bình thường. |
Không |
| · Giai đoạn C | ||||
| Hẹp hai lá khít chưa có triệu chứng | · Tổn thương van do thấp với dính mép van và các lá van mở dạng vòm trong thì tâm trương.
· Diện tích lỗ van ≤ 1,5 cm2 đo viền trực tiếp. |
· Diện tích lỗ van ≤ 1,5 cm2.
· Thời gian bán giảm áp lực tâm trương ≥ 150 ms |
· Nhĩ trái giãn nhiều.
· Tăng áp lực động mạch phổi tâm thu trên 50 mmHg. |
Không |
| · Giai đoạn D | ||||
| Hẹp hai lá khít có triệu chứng | · Tổn thương van do thấp với dính mép van và các lá van mở dạng vòm trong thì tâm trương.
· Diện tích lỗ van ≤ 1,5 cm2 đo viền trực tiếp. |
· Diện tích lỗ van ≤ 1,5 cm2.
· Thời gian bán giảm áp lực tâm trương ≥ 150 ms |
· Nhĩ trái giãn nhiều.
· Tăng áp lực động mạch phổi tâm thu trên 50 mmHg. |
· Giảm dung nạp gắng sức.
· Khó thở khi gắng sức. |
Cần đo chênh áp trung bình qua van hai lá để đánh giá ảnh hưởng về huyết đọng của hẹp van hai lá, chênh áp trung bình thường ≥ 5 mmHg tới 10 mmHg trong hẹp hái khít; tuy nhiên do chênh áp trung bình bị thay đổi do tần số tim và dòng chảy qua van hai lá nên thông số này không được đưa vào tiêu chuẩn phân độ.
Trừ huyết khối nhĩ trái trước trước can thiệp nong van hoặc sau khi có các biến cố thuyên tắc do huyết khối, và thu thập thêm thông tin về giải phẫu van hai lá (vùng mép van và bộ máy dưới van) trước can thiệp nếu chất lượng hình ảnh siêu âm tim qua thành ngực không tối ưu. Các nghiệm pháp gắng sức được chỉ định nếu bệnh nhân không có triệu chứng hoặc các triệu chứng không tương xứng với mức độ hẹp van. Siêu âm tim gắng sức thể lực có thể cung cấp thêm các thông tin khách quan hơn siêu âm tim gắng sức với dobutamin thông qua sự thay đổi chênh áp qua van hai lá và áp lực động mạch phổi. Siêu âm tim đóng vai trò quan trọng để theo dõi liên tục trong và sau can thiệp cũng như theo dõi người bệnh dài hạn.
12.2.2. Chỉ định can thiệp
Phương pháp can thiệp (nong van hay phẫu thuật), cũng như thời gian thích hợp nên được lựa chọn dựa trên đặc điểm lâm sàng, giải phẫu của van và bộ máy dưới van và ý kiến chuyên gia. Nhìn chung, can thiệp chỉ nên tiến hành ở bệnh nhân hẹp hai lá có ý nghĩa lâm sàng (hẹp hai lá mức độ vừa đến khít) do nguyên nhân hậu thấp (diện tích van ≤ 1,5 cm2) bởi can thiệp có hiệu quả đáng kể ở nhóm bệnh nhân này. Ở các nước phát triển, nơi tỷ lệ hẹp van hai lá do thấp khớp và số ca nong van hai lá thấp, chỉ nên tiến hành nong van bởi với các chuyên gia để tăng tính an toàn và tỷ lệ thành công của thủ thuât. Ở các nước đang phát triển cần nỗ lực giúp người bệnh được nong van hai lá bởi khả năng tiếp cận điều trị khó khăn do nguyên nhân kinh tế. Nong van hai lá qua da nên là lựa chọn ban đầu cho các bệnh nhân có mức độ vôi hóa và tổn thương bộ máy dưới van mức độ nhẹ đến vừa và có những đặc điểm lâm sàng phù hợp.
Quản lý bệnh nhân hẹp khít van hai lá do thấp tim được tóm tắt trong Hình 11, chỉ định và chống chỉ định nong van hai lá qua da tóm tắt ở Bảng 18.
12.2.3. Điều trị nội khoa
Thuốc lợi tiểu, chẹn beta giao cảm, digoxin, chẹn kênh canxi nhóm non-dihydrolpyridine, và ivabradine có thể cải thiện triệu chứng. Thuốc chống đông kháng vitamin K với mục tiêu INR 2 – 3 được chỉ định với các bệnh nhân rung nhĩ. Bệnh nhân hẹp hai lá mức độ vừa đến khít có rung nhĩ nên duy trì chống đông kháng vitamin K thay vì các thuốc chống đông đường uống thế hệ mới (NOACs). Hiện nay chưa có các bằng chứng thuyết phục về việc sử dụng NOACs trong trường hợp này với một thử nghiệm lâm sàng ngẫu nhiên đang bắt đầu được tiến hành (INVICTUS VKA NCT 02832544). Các biện pháp sốc điện chuyển nhịp hay cô lập tĩnh mạch phổi đều không được chỉ định trước khi can thiệp nong van hai lá ở bệnh nhân có hẹp hai lá đáng kể vì chúng không có tác dụng duy trì nhịp xoang lâu dài. Nếu rung nhĩ mới xuất hiện và nhĩ trái chỉ giãn mức độ vừa, nên tiến hành sốc điện chuyển nhịp sớm sau khi can thiệp thành công, cũng nên xem xét chuyển nhịp ở các bệnh nhân chưa đến mức hẹp van hai lá khít. Amiodarone là thuốc hiệu quả nhất trong duy trì nhịp tim sau chuyển nhịp. Ở bệnh nhân nhịp xoang, thuốc chống đông đường uống được khuyến cáo sử dụng trong trường hợp bệnh nhân có các biến cố tắc mạch hệ thống hoặc có huyết khối trong nhĩ trái và nên thực hiện siêu âm qua thức quản ở các trường hợp có nhiều âm cuộn trong siêu âm hoặc nhĩ trái giãn lớn (đường kính nhĩ trái đo trên siêu âm M-mode trên 50 mm hoặc chỉ số thể tích nhĩ trái trên 60 ml/m2).
12.2.4. Theo dõi
Bệnh nhân hẹp hai lá có ý nghĩa nhưng không có triệu chứng nên được theo dõi lâm sàng và siêu âm tim định kỳ mỗi năm, thời khoảng theo dõi có thể dài hơn (2 – 3 năm) trong các trường hợp hẹp vừa. Theo dõi các bệnh nhân sau nong van thành công tương tự các bệnh nhân không triệu chứng, nên theo dõi thường xuyên hơn nếu tái hẹp xảy ra.
12.2.5. Các trường hợp đặc biệt
Nếu các trường hợp tái hẹp xảy ra sau phẫu thuật hoặc sau nong van qua da, tái can thiệp trong hầu hết các trường hợp là thay van, tuy nhiên có thể nong van qua da đối với một số bệnh nhân phù hợp nếu cơ chế tái hẹp chủ yếu là dính lại mép van.
Ở các bệnh nhân có hẹp hai lá khít kết hợp với tổn thương van động mạch chủ nặng, phẫu thuật được ưu tiên hơn nếu bệnh nhân không có chống chỉ định với phẫu thuật. Quản lý các bệnh nhân chống chỉ định với phẫu thuật là khó khăn và yêu cầu sự đánh giá chuyên sâu, cá nhân hóa của nhóm chuyên gia tim mạch (heart team). Trong trường hợp hẹp hai lá khít, tổn thương van động mạch chủ mức độ vừa, nong van hai lá qua da có thể được tiến hành để trì hoãn cho đến khi phẫu thuật sửa chữa cả hai van. Ở các bệnh nhân có hở van ba nhiều, có thể xem xét nong van hai lá qua da ở các bệnh nhân vẫn duy trì được nhịp xoang, giãn nhĩ trái mức độ vừa, hở ba lá nhiều thứ phát do tăng áp động mạch phổi. Trong các trường hợp khác, nên phẫu thuật cả hai van.
Ở người già có hẹp van hai lá do thấp, nếu nguy cơ phẫu thuật cao, nong van hai lá qua da là lựa chọn cần thiết, thậm chí là chỉ chăm sóc giảm nhẹ. Điều trị cho bệnh nhân hẹp van hai lá khít chênh áp thấp (diện tích lỗ van ≤ 1,5 cm2 và chênh áp trung bình dưới 10 mmHg) là vấn đề khó bởi các bệnh nhân này thường già và giải phẫu van thường không lý tưởng.
13. Hẹp van hai lá do vôi hóa vòng van
13.1. Đánh giá
Ở các bệnh nhân có thoái hóa và vôi hóa vòng van van hai lá, khó đánh giá mức độ nặng của van trên siêu âm tim và các phép đo thường quy thiếu tính xác thực (lack validation). Đo viền diện tích lỗ van (planimetry) thường thiếu tin cậy do sự vôi hóa và hình thái lỗ van không đều. Chênh áp trung bình qua van có ý nghĩa tiên lượng. Để đánh giá mức độ hẹp, cần đánh giá cả tình trạng rối loạn khả năng giãn (compliance) của thất trái và nhĩ trái trước khi chỉ định can thiệp. Nếu bệnh nhân có kế hoạch can thiệp, siêu âm tim thường được sử dụng để đánh giá ban đầu và cần chụp cắt lớp vi tính là để đánh giá mức độ và vị trí vôi hóa và mức độ phù hợp để can thiệp.
13.2. Chỉ định can thiệp
Lựa chọn điều trị, bao gồm phẫu thuật và can thiệp qua đường ống thông đều là thủ thuật nguy cơ cao, và thiếu bằng chứng từ các thử nghiệm ngẫu nhiên. Thậm chí nếu thủ thuật thành công và chênh áp qua van giảm, áp lực trung bình nhĩ trái vẫn tiếp tục tăng do giảm khả năng giãn (low compliance) của nhĩ trái và thất trái.
Với những người lớn tuổi có thoái hóa van hai lá và vôi hóa vòng van, phẫu thuật có tính thách thức cao về mặt kỹ thuật và nguy cơ cao. Vì không có tổn thương mép van, hẹp hai lá do thoái hóa không phù hợp để nong van hai lá qua da. Với những bệnh nhân có triệu chứng không thể phẫu thuật và có giải phẫu van phù hợp, kinh nghiệm thực tế cho thấy thay van hai lá qua da (bằng cách sử dụng van TAVI tự nở trên bóng nhưng đảo chiều để đặt vào vị trí van hai lá) có thể thực hiện được ở một số bệnh nhân hẹp hai lá khít phù hợp, khi được thực hiên tại các trung tâm có nhiều kinh nghiệm sau khi có kế hoạch cẩn thận và phối hợp nhiều phương tiện chẩn đoán hình ảnh. Chuỗi các ca lâm sàng nhiều nhất đến nay chỉ bao gồm 116 bệnh nhân. Tuy nhiên, tỷ lệ tử vong cao đặc biệt là do nguy cơ gây tắc nghẽn đường ra thất trái, và tiên lượng trung hạn cũng kém hơn can thiệp đặt van trong van. Chuỗi ca lâm sàng gần đây cho thấy các kết quả đã được cải thiện do các bệnh nhân được lựa chọn cẩn thận và sử dụng các phương pháp tiếp cận khác nhau, cũng như cùng sử dụng các biện pháp khác như đốt cồn vách liên thất cùng lúc hoặc dự phòng hoặc cắt bỏ lá trước van hai lá.
Gần đây, một chuỗi các ca lâm sàng sơ bộ gợi ý rằng thay van hai lá qua đường ống thông với van hai lá nhân tạo chuyên dụng có tính khả thi và có thể cải thiện triệu chứng.
Khuyến cáo về chỉ định nong van hai lá qua ống thông và phẫu thuật van hai lá ở bệnh nhân hẹp khít van hai lá (diện tích van ≤ 1,5 cm2) theo ESC năm 2021(18).
| Loại | MCC | Khuyến cáo |
| I | B | 1. Nong van hai lá qua da được khuyến cáo ở các bệnh nhân có triệu chứng và không có chống chỉ định cho nong van |
| I | C | 2. Nong van hai lá qua da được khuyến cáo ở các bệnh nhân có triệu chứng có chống chỉ định hoặc nguy cơ phẫu thuật cao |
| I | C | 3. Phẫu thuật van hai lá được khuyến cáo với các bệnh nhân có triệu chứng, không phù hợp để nong van in |
| IIa | C | 4. Nong van hai lá qua da nên được xem xét như lựa chọn ban đầu ở các bệnh nhân có triệu chứng mặc dù hình thái van không tối ưu nhất nhưng không có các đặc điểm khác không phù hợp với nong van hai lá qua da. |
| IIa | C | 5. Nong van hai lá qua da nên được xem xét ở các bệnh nhân không triệu chứng không có các đặc điểm lâm sàng hay các đặc điểm hình thái van không phù hợp với nong van và
Nguy cơ huyết khối cao (tiền sử huyết khối hệ thống, âm cuộn dày trong nhĩ trái, rung nhĩ mới hay rung nhĩ kịch phát) và/hoặc Nguy cơ mất bù huyết động (áp lực động mạch phổi trên 50mmHg lúc nghỉ, chuẩn bị cho các phẫu thuật không tim mạch lớn, mong muốn có thai) |
Các đặc điểm không phù hợp cho nong van hai lá qua da được định nghĩa bởi sự có mặt của một số đặc điểm dưới đây. Đặc điểm lâm sàng bao gồm: tuổi cao, tiền sử phẫu thuật tách van, khó thở NYHA IV, rung nhĩ dai dẳng, tăng áp động mạch phổi nhiều. Các đặc điểm giải phẫu van: Điểm siêu âm tim trên 8, điểm Cormier 3 (vôi hóa van hai lá mở rộng đánh giá bới nội soi huỳnh quang), diện tích nhĩ trái rất nhỏ, hở van ba lá nhiều.
Bảng 18. Chống chỉ định nong van hai lá qua da ở bệnh nhân hẹp hai lá khít do thấp
| Chống chỉ định |
| Diện tích van hai lá trên 1,5 cm2 a |
| Huyết khối nhĩ trái |
| Hở van hai lá từ mức độ vừa trở lên |
| Vôi hóa nặng hoặc vôi cả hai mép van |
| Không có tình trạng dính hai mép van |
| Kèm theo tổn thương nặng van động mạch chủ, hoặc kèm theo hẹp hở van ba lá nặng cần phẫu thuật |
| Kèm tổn thương mạch vành cần phẫu thuật bắc cầu nối chủ bành |
a Nong van hai lá qua da có thể xem xét ở bệnh nhân có diện tích van trên 1,5 cm2 có triệu chứng không thể lý giải bởi các các nguyên nhân khác và giải phẫu phù hợp để nong van.
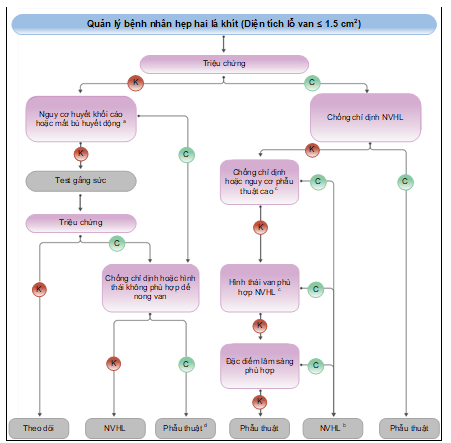
Hình 11. Quản lý bệnh nhân hẹp hai lá khít (diện tích lỗ van ≤ 1,5 cm2) theo ESC 2021(18)
a Nguy cơ huyết khối cao: Tiền sử có huyết khối hệ thống, âm cuộn đặc trong nhĩ trái, rung nhĩ mới khởi phát. Nguy cơ cao huyết động mát bù: áp lức động mạch phổi trên 50 mmHg lúc nghỉ, cần phẫu thuật ngoài tim mạch lớn, mong muốn có thai.
b Phẫu thuật tách van có thể xem xét ở các nhóm phẫu thuật có kinh nghiệm với các bệnh nhân có chống chỉ định với NVHL qua da.
c Xem khuyến cáo chỉ định cho NVHL và phẫu thuật van hai lá xem ở mục 7.2.
d Phẫu thuật nếu các triệu chứng xuất hiện khi gắng sức mức độ thấp và nguy cơ phẫu thuật thấp.
NVH: nong van hai lá.
TÀI LIỆU THAM KHẢO
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25-e197. https://doi.org/10.1016/j.jacc.2020.11.018
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22(1):1-23. https://doi.org/10.1016/J.ECHO.2008.11.029
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010;11(4):307-332. https://doi.org/10.1093/EJECHOCARD/JEQ031
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303-371. https://doi.org/10.1016/J.ECHO.2017.01.007
- Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(4):372-392. https://doi.org/10.1016/J.ECHO.2017.02.009
- Currie PJ, Seward JB, Reeder GS, et al. Continuous-wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous Doppler-catheter correlative study in 100 adult patients. Circulation. 1985;71(6):1162-1169. https://doi.org/10.1161/01.CIR.71.6.1162
- Medvedofsky D, Maffessanti F, Weinert L, et al. 2D and 3D Echocardiography-Derived Indices of Left Ventricular Function and Shape: Relationship With Mortality. JACC Cardiovasc Imaging. 2018;11(11):1569-1579. https://doi.org/10.1016/J.JCMG.2017.08.023
- Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167-184. https://doi.org/10.1067/MJE.2002.120202
- Habib G, Lancellotti P. The 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J. 2015;36(44):3036-3037. https://doi.org/10.1093/EURHEARTJ/EHV488
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111(24):3290-3295. https://doi.org/10.1161/CIRCULATIONAHA.104.495903
- Rosenhek R, Iung B, Tornos P, et al. ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J. 2012;33(7). https://doi.org/10.1093/EURHEARTJ/EHR061
- Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6(7). https://doi.org/10.1161/JAHA.117.005835
- Lip GYH, Jensen M, Melgaard L, Skjøth F, Nielsen PB, Larsen TB. Stroke and bleeding risk scores in patients with atrial fibrillation and valvular heart disease: evaluating “valvular heart disease” in a nationwide cohort study. Europace. 2019;21(1):33-40. https://doi.org/10.1093/EUROPACE/EUY151
- Vora AN, Dai D, Matsuoka R, et al. Incidence, Management, and Associated Clinical Outcomes of New-Onset Atrial Fibrillation Following Transcatheter Aortic Valve Replacement: An Analysis From the STS/ACC TVT Registry. JACC Cardiovasc Interv. 2018;11(17):1746-1756. https://doi.org/10.1016/J.JCIN.2018.05.042
- Seeger J, Gonska B, Rodewald C, Rottbauer W, Wöhrle J. Apixaban in Patients With Atrial Fibrillation After Transfemoral Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10(1):66-74. https://doi.org/10.1016/J.JCIN.2016.10.023
- Jochheim D, Barbanti M, Capretti G, et al. Oral Anticoagulant Type and Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12(16):1566-1576. https://doi.org/10.1016/J.JCIN.2019.03.003
- JW E, SJ C, M B, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13). https://doi.org/10.1056/NEJMOA1300615
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561-632. https://doi.org/10.1093/eurheartj/ehab395
- Aicher D, Kunihara T, Abou Issa O, Brittner B, Gräber S, Schäfers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123(2):178-185. https://doi.org/10.1161/CIRCULATIONAHA.109.934679
- David TE, Armstrong S, Ivanov J, Webb GD. Aortic valve sparing operations: an update. Ann Thorac Surg. 1999;67(6):1840-1842. https://doi.org/10.1016/S0003-4975(99)00420-8
- Kallenbach K, Hagl C, Walles T, et al. Results of valve-sparing aortic root reconstruction in 158 consecutive patients. Ann Thorac Surg. 2002;74(6):2026-2033. https://doi.org/10.1016/S0003-4975(02)04090-0
- Pettersson GB, Crucean AC, Savage R, et al. Toward Predictable Repair of Regurgitant Aortic Valves: A Systematic Morphology-Directed Approach to Bicommissural Repair. J Am Coll Cardiol. 2008;52(1):40-49. https://doi.org/10.1016/J.JACC.2008.01.073
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg. 2011;142(6):1430-1438. https://doi.org/10.1016/J.JTCVS.2011.08.021
- Kari FA, Liang DH, Escobar Kvitting JP, et al. Tirone David valve-sparing aortic root replacement and cusp repair for bicuspid aortic valve disease. J Thorac Cardiovasc Surg. 2013;145(3):S35-S40.e2. https://doi.org/10.1016/J.JTCVS.2012.11.043
- Ouzounian M, Rao V, Manlhiot C, et al. Valve-Sparing Root Replacement Compared With Composite Valve Graft Procedures in Patients With Aortic Root Dilation. J Am Coll Cardiol. 2016;68(17):1838-1847. https://doi.org/10.1016/J.JACC.2016.07.767
- Lang RM, Badano LP, Victor MA, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2015;28(1):1-39.e14. https://doi.org/10.1016/J.ECHO.2014.10.003
- Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. Journal of Cardiovascular Magnetic Resonance. 2015;17(1):1-33. https://doi.org/10.1186/S12968-015-0111-7/COMMENTS
- Bonow RO, Borer JS, Rosing DR, et al. Preoperative exercise capacity in symptomatic patients with aortic regurgitation as a predictor of postoperative left ventricular function and long-term prognosis. Circulation. 1980;62(6):1280-1290. https://doi.org/10.1161/01.CIR.62.6.1280
- Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Optimizing Timing of Surgical Correction in Patients With Severe Aortic Regurgitation: Role of Symptoms. J Am Coll Cardiol. 1997;30(3):746-752. https://doi.org/10.1016/S0735-1097(97)00205-2
- Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002;106(21):2687-2693. https://doi.org/10.1161/01.CIR.0000038498.59829.38
- Tornos P, Sambola A, Permanyer-Miralda G, Evangelista A, Gomez Z, Soler-Soler J. Long-Term Outcome of Surgically Treated Aortic Regurgitation: Influence of Guideline Adherence Toward Early Surgery. J Am Coll Cardiol. 2006;47(5):1012-1017. https://doi.org/10.1016/J.JACC.2005.10.049
- Bhudia SK, McCarthy PM, Kumpati GS, et al. Improved Outcomes After Aortic Valve Surgery for Chronic Aortic Regurgitation With Severe Left Ventricular Dysfunction. J Am Coll Cardiol. 2007;49(13):1465-1471. https://doi.org/10.1016/J.JACC.2007.01.026
- Fiedler AG, Bhambhani V, Laikhter E, et al. Aortic valve replacement associated with survival in severe regurgitation and low ejection fraction. Heart. 2018;104(10):835-840. https://doi.org/10.1136/HEARTJNL-2017-312024
- Kaneko T, Ejiofor JI, Neely RC, et al. Aortic Regurgitation With Markedly Reduced Left Ventricular Function Is Not a Contraindication for Aortic Valve Replacement. Ann Thorac Surg. 2016;102(1):41-47. https://doi.org/10.1016/J.ATHORACSUR.2015.12.068
- Forman R, Firth BG, Barnard MS. Prognostic significance of preoperative left ventricular ejection fraction and valve lesion in patients with aortic valve replacement. Am J Cardiol. 1980;45(6):1120-1125. https://doi.org/10.1016/0002-9149(80)90468-3
- Bonow RO, Picone AL, McIntosh CL, et al. Survival and functional results after valve replacement for aortic regurgitation from 1976 to 1983: impact of preoperative left ventricular function. Circulation. 1985;72(6):1244-1256. https://doi.org/10.1161/01.CIR.72.6.1244
- Cormier B, Vahanian A, Luxereau P, Kassab R, Acar J. Should asymptomatic or mildly symptomatic aortic regurgitation be operated on? Z Kardiol. 1986;75:141-145.
- Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Aortic regurgitation complicated by extreme left ventricular dilation: long-term outcome after surgical correction. J Am Coll Cardiol. 1996;27(3):670-677. https://doi.org/10.1016/0735-1097(95)00525-0
- Saisho H, Arinaga K, Kikusaki S, et al. Long Term Results and Predictors of Left Ventricular Function Recovery after Aortic Valve Replacement for Chronic Aortic Regurgitation. Annals of Thoracic and Cardiovascular Surgery. 2015;21(4):388-395. https://doi.org/10.5761/ATCS.OA.14-00295
- Mentias A, Feng K, Alashi A, et al. Long-Term Outcomes in Patients With Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2016;68(20):2144-2153. https://doi.org/10.1016/J.JACC.2016.08.045
- Yang LT, Michelena HI, Scott CG, et al. Outcomes in Chronic Hemodynamically Significant Aortic Regurgitation and Limitations of Current Guidelines. J Am Coll Cardiol. 2019;73(14):1741-1752. https://doi.org/10.1016/J.JACC.2019.01.024
- de Meester C, Gerber BL, Vancraeynest D, et al. Do Guideline-Based Indications Result in an Outcome Penalty for Patients With Severe Aortic Regurgitation? JACC Cardiovasc Imaging. 2019;12(11):2126-2138. https://doi.org/10.1016/J.JCMG.2018.11.022
- Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84(4):1625-1635. https://doi.org/10.1161/01.CIR.84.4.1625
- Pizarro R, Bazzino OO, Oberti PF, et al. Prospective Validation of the Prognostic Usefulness of B-Type Natriuretic Peptide in Asymptomatic Patients With Chronic Severe Aortic Regurgitation. J Am Coll Cardiol. 2011;58(16):1705-1714. https://doi.org/10.1016/J.JACC.2011.07.016
- Tornos MP, Olona M, Permanyer-Miralda G, et al. Clinical outcome of severe asymptomatic chronic aortic regurgitation: A long-term prospective follow-up study. Am Heart J. 1995;130(2):333-339. https://doi.org/10.1016/0002-8703(95)90450-6
- Tarasoutchi F, Grinberg M, Spina GS, et al. Ten-year clinical laboratory follow-up after application of a symptom-based therapeutic strategy to patients with severe chronic aortic regurgitation of predominant rheumatic etiology. J Am Coll Cardiol. 2003;41(8):1316-1324. https://doi.org/10.1016/S0735-1097(03)00129-3
- Kumpuris AG, Quinones MA, Waggoner AD, Kanon DJ, Nelson JG, Miller RR. Importance of preoperative hypertrophy, wall stress and end-systolic dimension as echocardiographic predictors of normalization of left ventricular dilatation after valve replacement in chronic aortic insufficiency. Am J Cardiol. 1982;49(5):1091-1100. https://doi.org/10.1016/0002-9149(82)90032-7
- Fioretti P, Roelandt J, Bos RJ, et al. Echocardiography in chronic aortic insufficiency. Is valve replacement too late when left ventricular end-systolic dimension reaches 55 mm? Circulation. 1983;67(1):216-221. https://doi.org/10.1161/01.CIR.67.1.216
- Detaint D, Messika-Zeitoun D, Maalouf J, et al. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. JACC Cardiovasc Imaging. 2008;1(1):1-11. https://doi.org/10.1016/J.JCMG.2007.10.008
- Stone PH, Clark RD, Goldschlager N, Selzer A, Cohn K. Determinants of prognosis of patients with aortic regurgitation who undergo aortic valve replacement. J Am Coll Cardiol. 1984;3(5):1118-1126. https://doi.org/10.1016/S0735-1097(84)80168-0
- Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Aortic regurgitation complicated by extreme left ventricular dilation: Long-term outcome after surgical correction. J Am Coll Cardiol. 1996;27(3):670-677. https://doi.org/10.1016/0735-1097(95)00525-0
- Zhang Z, Yang J, Yu Y, et al. Preoperative ejection fraction determines early recovery of left ventricular end-diastolic dimension after aortic valve replacement for chronic severe aortic regurgitation. Journal of Surgical Research. 2015;196(1):49-55. https://doi.org/10.1016/J.JSS.2015.02.069
- Murashita T, Schaff H v., Suri RM, et al. Impact of Left Ventricular Systolic Function on Outcome of Correction of Chronic Severe Aortic Valve Regurgitation: Implications for Timing of Surgical Intervention. Ann Thorac Surg. 2017;103(4):1222-1228. https://doi.org/10.1016/J.ATHORACSUR.2016.09.004
- Wang Y, Jiang W, Liu J, et al. Early surgery versus conventional treatment for asymptomatic severe aortic regurgitation with normal ejection fraction and left ventricular dilatation. Eur J Cardiothorac Surg. 2017;52(1):118-124. https://doi.org/10.1093/EJCTS/EZX018
- Scognamiglio R, Rahimtoola SH, Fasoli G, Nistri S, Volta SD. Nifedipine in asymptomatic patients with severe aortic regurgitation and normal left ventricular function. N Engl J Med. 1994;331(11):689-694. https://doi.org/10.1056/NEJM199409153311101
- Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart. 2017;103(17):1323-1330. https://doi.org/10.1136/HEARTJNL-2016-309916
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44(1):138-143. https://doi.org/10.1016/J.JACC.2004.03.050
- Huntington K, Hunter AGW, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol. 1997;30(7):1809-1812. https://doi.org/10.1016/S0735-1097(97)00372-0
- Glick BN, Roberts WC. Congenitally bicuspid aortic valve in multiple family members. Am J Cardiol. 1994;73(5):400-404. https://doi.org/10.1016/0002-9149(94)90018-3
- Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: A disorder of uncertain inheritance. Am J Med Genet. 1996;62(4):336-338. https://doi.org/10.1002/(SICI)1096-8628(19960424)62:4<336::AID-AJMG2>3.0.CO;2-P
- Kong WKF, Delgado V, Bax JJ. Bicuspid Aortic Valve: What to Image in Patients Considered for Transcatheter Aortic Valve Replacement? Circ Cardiovasc Imaging. 2017;10(9). https://doi.org/10.1161/CIRCIMAGING.117.005987
- Michelena HI, Prakash SK, Corte A della, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 2014;129(25):2691-2704. https://doi.org/10.1161/CIRCULATIONAHA.113.007851
- Elefteriades JA, Sang A, Kuzmik G, Hornick M. Guilt by association: paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart. 2015;2(1). https://doi.org/10.1136/OPENHRT-2014-000169
- Burstow DJ, Nishimura RA, Bailey KR, et al. Continuous wave Doppler echocardiographic measurement of prosthetic valve gradients. A simultaneous Doppler-catheter correlative study. Circulation. 1989;80(3):504-514. https://doi.org/10.1161/01.CIR.80.3.504
- Dumesnil JG, Honos GN, Lemieux M, Beauchemin J. Validation and applications of indexed aortic prosthetic valve areas calculated by Doppler echocardiography. J Am Coll Cardiol. 1990;16(3):637-643. https://doi.org/10.1016/0735-1097(90)90355-S
- Baumgartner H, Khan S, DeRobertis M, Czer L, Maurer G. Effect of prosthetic aortic valve design on the Doppler-catheter gradient correlation: an in vitro study of normal St. Jude, Medtronic-Hall, Starr-Edwards and Hancock valves. J Am Coll Cardiol. 1992;19(2):324-332. https://doi.org/10.1016/0735-1097(92)90486-7
- Vandervoort PM, Greenberg NL, Pu M, Powell KA, Cosgrove DM, Thomas JD. Pressure recovery in bileaflet heart valve prostheses. Localized high velocities and gradients in central and side orifices with implications for Doppler-catheter gradient relation in aortic and mitral position. Circulation. 1995;92(12):3464-3472. https://doi.org/10.1161/01.CIR.92.12.3464
- Salaun E, Mahjoub H, Girerd N, et al. Rate, Timing, Correlates, and Outcomes of Hemodynamic Valve Deterioration After Bioprosthetic Surgical Aortic Valve Replacement. Circulation. 2018;138(10):971-985. https://doi.org/10.1161/CIRCULATIONAHA.118.035150
- Rahimtoola SH. Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. 2010;55(22):2413-2426. https://doi.org/10.1016/J.JACC.2009.10.085
- van Geldorp MWA, Eric Jamieson WR, Kappetein AP, et al. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137(4). https://doi.org/10.1016/J.JTCVS.2008.09.028
- Salaun E, Mahjoub H, Dahou A, et al. Hemodynamic Deterioration of Surgically Implanted Bioprosthetic Aortic Valves. J Am Coll Cardiol. 2018;72(3):241-251. https://doi.org/10.1016/J.JACC.2018.04.064
- Douglas PS, Leon MB, Mack MJ, et al. Longitudinal Hemodynamics of Transcatheter and Surgical Aortic Valves in the PARTNER Trial. JAMA Cardiol. 2017;2(11):1197-1206. https://doi.org/10.1001/JAMACARDIO.2017.3306
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477-2484. https://doi.org/10.1016/S0140-6736(15)60308-7
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2485-2491. https://doi.org/10.1016/S0140-6736(15)60290-2
- Fernández-Santos S, Théron A, Pibarot P, et al. Valve hemodynamic performance and myocardial strain after implantation of a third-generation, balloon-expandable, transcatheter aortic valve. Cardiol J. 2020;27(6):789-796. https://doi.org/10.5603/CJ.A2019.0049
- Manoharan G, van Mieghem NM, Windecker S, et al. 1-Year Outcomes With the Evolut R Self-Expanding Transcatheter Aortic Valve: From the International FORWARD Study. JACC Cardiovasc Interv. 2018;11(22):2326-2334. https://doi.org/10.1016/J.JCIN.2018.07.032
- Gleason TG, Reardon MJ, Popma JJ, et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J Am Coll Cardiol. 2018;72(22):2687-2696. https://doi.org/10.1016/J.JACC.2018.08.2146
- Blackman DJ, Saraf S, MacCarthy PA, et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses. J Am Coll Cardiol. 2019;73(5):537-545. https://doi.org/10.1016/J.JACC.2018.10.078
- Søndergaard L, Ihlemann N, Capodanno D, et al. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J Am Coll Cardiol. 2019;73(5):546-553. https://doi.org/10.1016/J.JACC.2018.10.083
- Kaneko T, Aranki S, Javed Q, et al. Mechanical versus bioprosthetic mitral valve replacement in patients. J Thorac Cardiovasc Surg. 2014;147(1):117-126. https://doi.org/10.1016/J.JTCVS.2013.08.028
- Bourguignon T, Bouquiaux-Stablo AL, Loardi C, et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg. 2014;148(5):2004-2011.e1. https://doi.org/10.1016/J.JTCVS.2014.02.050
- Weber A, Noureddine H, Englberger L, et al. Ten-year comparison of pericardial tissue valves versus mechanical prostheses for aortic valve replacement in patients younger than 60 years of age. J Thorac Cardiovasc Surg. 2012;144(5):1075-1083. https://doi.org/10.1016/J.JTCVS.2012.01.024
- Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36(4):1152-1158. https://doi.org/10.1016/S0735-1097(00)00834-2
- Chan V, Jamieson WRE, Germann E, et al. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after aortic valve replacement. J Thorac Cardiovasc Surg. 2006;131(6):1267-1273. https://doi.org/10.1016/J.JTCVS.2005.11.052
- Banbury MK, Cosgrove DM, Thomas JD, et al. Hemodynamic stability during 17 years of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg. 2002;73(5):1460-1465. https://doi.org/10.1016/S0003-4975(02)03445-8
- Dellgren G, David TE, Raanani E, Armstrong S, Ivanov J, Rakowski H. Late hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2002;124(1):146-154. https://doi.org/10.1067/MTC.2002.121672
- Borger MA, Ivanov J. Twenty-Year Results of the Hancock II Bioprosthesis.; 2006. https://www.researchgate.net/publication/7297016
- Mykén PSU, Bech-Hansen O. A 20-year experience of 1712 patients with the Biocor porcine bioprosthesis. J Thorac Cardiovasc Surg. 2009;137(1):76-81. https://doi.org/10.1016/J.JTCVS.2008.05.068
- Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N Engl J Med. 2017;377(19):1847-1857. https://doi.org/10.1056/NEJMOA1613792
- Badhwar V, Ofenloch JC, Rovin JD, van Gelder HM, Jacobs JP. Noninferiority of closely monitored mechanical valves to bioprostheses overshadowed by early mortality benefit in younger patients. Ann Thorac Surg. 2012;93(3):748-753. https://doi.org/10.1016/J.ATHORACSUR.2011.12.032
- Brown ML, Schaff H v., Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg. 2008;135(4):878-884. https://doi.org/10.1016/J.JTCVS.2007.10.065
- Kulik A, Bédard P, Lam BK, et al. Mechanical versus bioprosthetic valve replacement in middle-aged patients. Eur J Cardiothorac Surg. 2006;30(3):485-491. https://doi.org/10.1016/J.EJCTS.2006.06.013
- Glaser N, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50-69 years. Eur Heart J. 2016;37(34):2658-2667. https://doi.org/10.1093/EURHEARTJ/EHV580
- Chikwe J, Chiang YP, Egorova NN, Itagaki S, Adams DH. Survival and outcomes following bioprosthetic vs mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA. 2015;313(14):1435-1442. https://doi.org/10.1001/JAMA.2015.3164
- McClure RS, McGurk S, Cevasco M, et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis. J Thorac Cardiovasc Surg. 2014;148(5):1931-1939. https://doi.org/10.1016/J.JTCVS.2013.12.042
- Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014;312(13):1323-1329. https://doi.org/10.1001/JAMA.2014.12679
- Buratto E, Shi WY, Wynne R, et al. Improved Survival After the Ross Procedure Compared With Mechanical Aortic Valve Replacement. J Am Coll Cardiol. 2018;71(12):1337-1344. https://doi.org/10.1016/J.JACC.2018.01.048
- El-Hamamsy I, Eryigit Z, Stevens LM, et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376(9740):524-531. https://doi.org/10.1016/S0140-6736(10)60828-8
- Martin E, Mohammadi S, Jacques F, et al. Clinical Outcomes Following the Ross Procedure in Adults: A 25-Year Longitudinal Study. J Am Coll Cardiol. 2017;70(15):1890-1899. https://doi.org/10.1016/J.JACC.2017.08.030
- Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJM, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333(1):11-17. https://doi.org/10.1056/NEJM199507063330103
- Sun JC, Davidson MJ, Lamy A, Eikelboom JW. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009;374(9689):565-576. https://doi.org/10.1016/S0140-6736(09)60780-7
- van de Werf F, Brueckmann M, Connolly SJ, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J. 2012;163(6). https://doi.org/10.1016/J.AHJ.2012.03.011
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89(2):635-641. https://doi.org/10.1161/01.CIR.89.2.635
- Torella M, Torella D, Chiodini P, et al. LOWERing the INtensity of oral anticoaGulant Therapy in patients with bileaflet mechanical aortic valve replacement: results from the “LOWERING-IT” Trial. Am Heart J. 2010;160(1):171-178. https://doi.org/10.1016/J.AHJ.2010.05.005
- Hering D, Piper C, Bergemann R, et al. Thromboembolic and bleeding complications following St. Jude Medical valve replacement: results of the German Experience With Low-Intensity Anticoagulation Study. Chest. 2005;127(1):53-59. https://doi.org/10.1378/CHEST.127.1.53
- Acar J, Iung B, Boissel JP, et al. AREVA: multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation. 1996;94(9):2107-2112. https://doi.org/10.1161/01.CIR.94.9.2107
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e576S-e600S. https://doi.org/10.1378/CHEST.11-2305
- Horstkotte D, Scharf RE, Schultheiss HP. Intracardiac thrombosis: patient-related and device-related factors. J Heart Valve Dis. 1995;4(2):114-120. Accessed May 30, 2022. https://europepmc.org/article/med/8556170
- Pruefer D, Dahm M, Dohmen G, Horstkotte D, Bergemann R, Oelert H. Intensity of oral anticoagulation after implantation of St. Jude Medical mitral or multiple valve replacement: lessons learned from GELIA (GELIA 5). European Heart Journal Supplements. 2001;3(suppl_Q):Q39-Q43. https://doi.org/10.1016/S1520-765X(01)90041-0
- Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc Interv. 2017;10(13):1357-1365. https://doi.org/10.1016/J.JCIN.2017.04.014
- Zuo W, Yang M, He Y, Hao C, Chen L, Ma G. Single or dual antiplatelet therapy after transcatheter aortic valve replacement: an updated systemic review and meta-analysis. J Thorac Dis. 2019;11(3):959-968. https://doi.org/10.21037/JTD.2019.01.87
- Maes F, Stabile E, Ussia GP, et al. Meta-Analysis Comparing Single Versus Dual Antiplatelet Therapy Following Transcatheter Aortic Valve Implantation. Am J Cardiol. 2018;122(2):310-315. https://doi.org/10.1016/J.AMJCARD.2018.04.006
- Heras M, Chesebro JH, Fuster V, et al. High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol. 1995;25(5):1111-1119. https://doi.org/10.1016/0735-1097(94)00563-6
- Colli A, Castella M. Comparing Warfarin to Aspirin (WoA) after Aortic Valve Replacement with the St. Jude Medical EpicTM Heart Valve Bioprosthesis: Results of the WoA Epic Pilot Trial. Published online 2007. Accessed May 31, 2022. https://www.researchgate.net/publication/5752586
- Aramendi JI, Mestres CA, Martinez-León J, Campos V, Muñoz G, Navas C. Triflusal versus oral anticoagulation for primary prevention of thromboembolism after bioprosthetic valve replacement (trac): prospective, randomized, co-operative trial. Eur J Cardiothorac Surg. 2005;27(5):854-860. https://doi.org/10.1016/J.EJCTS.2004.12.064
- Nuñez L, Aguado MG, Larrea JL, Celemín D, Oliver J. Prevention of thromboembolism using aspirin after mitral valve replacement with porcine bioprosthesis. Ann Thorac Surg. 1984;37(1):84-87. https://doi.org/10.1016/S0003-4975(10)60717-5
- Tiede DJ, Nishimura RA, Gastineau DA, Mullany CJ, Orszulak TA, Schaff H v. Modern management of prosthetic valve anticoagulation. Mayo Clin Proc. 1998;73(7):665-680. https://doi.org/10.1016/S0025-6196(11)64893-3
- Mérie C, Køber L, Skov Olsen P, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA. 2012;308(20):2118-2125. https://doi.org/10.1001/JAMA.2012.54506
- Russo A, Grigioni F, Avierinos JF, et al. Thromboembolic complications after surgical correction of mitral regurgitation incidence, predictors, and clinical implications. J Am Coll Cardiol. 2008;51(12):1203-1211. https://doi.org/10.1016/J.JACC.2007.10.058
- Egbe AC, Pislaru S v., Pellikka PA, et al. Bioprosthetic Valve Thrombosis Versus Structural Failure: Clinical and Echocardiographic Predictors. J Am Coll Cardiol. 2015;66(21):2285-2294. https://doi.org/10.1016/J.JACC.2015.09.022
- Sundt TM, Zehr KJ, Dearani JA, et al. Is early anticoagulation with warfarin necessary after bioprosthetic aortic valve replacement? J Thorac Cardiovasc Surg. 2005;129(5):1024-1031. https://doi.org/10.1016/J.JTCVS.2004.11.028
- ElBardissi AW, DiBardino DJ, Chen FY, Yamashita MH, Cohn LH. Is early antithrombotic therapy necessary in patients with bioprosthetic aortic valves in normal sinus rhythm? J Thorac Cardiovasc Surg. 2010;139(5):1137-1145. https://doi.org/10.1016/J.JTCVS.2009.10.064
- Massel DR, Little SH. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2013;2013(7). https://doi.org/10.1002/14651858.CD003464.PUB2
- Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014;147(4). https://doi.org/10.1016/J.JTCVS.2014.01.004
- Puskas JD, Gerdisch M, Nichols D, et al. Anticoagulation and Antiplatelet Strategies After On-X Mechanical Aortic Valve Replacement. J Am Coll Cardiol. 2018;71(24):2717-2726. https://doi.org/10.1016/J.JACC.2018.03.535
- Ussia GP, Scarabelli M, Mul M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2011;108(12):1772-1776. https://doi.org/10.1016/J.AMJCARD.2011.07.049
- Chakravarty T, Patel A, Kapadia S, et al. Anticoagulation After Surgical or Transcatheter Bioprosthetic Aortic Valve Replacement. J Am Coll Cardiol. 2019;74(9):1190-1200. https://doi.org/10.1016/J.JACC.2019.06.058
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N Engl J Med. 2015;373(21):2015-2024. https://doi.org/10.1056/NEJMOA1509233
- Jose J, Sulimov DS, El-Mawardy M, et al. Clinical Bioprosthetic Heart Valve Thrombosis After Transcatheter Aortic Valve Replacement: Incidence, Characteristics, and Treatment Outcomes. JACC Cardiovasc Interv. 2017;10(7):686-697. https://doi.org/10.1016/J.JCIN.2017.01.045
- Dangas GD, Tijssen JGP, Wöhrle J, et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N Engl J Med. 2020;382(2):120-129. https://doi.org/10.1056/NEJMOA1911425
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. https://doi.org/10.1056/NEJMOA0905561
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. https://doi.org/10.1056/NEJMOA1310907
- Summary of the article: Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med, 2011; 365: 1557–1559 | Szczerba | Kardiologia Polska (Polish Heart Journal). Accessed June 1, 2022. https://journals.viamedica.pl/kardiologia_polska/article/view/79117
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2011;365(11):981-992. https://doi.org/10.1056/NEJMOA1107039/SUPPL_FILE/NEJMOA1107039_DISCLOSURES.PDF
- Edmunds LH. Thrombotic and bleeding complications of prosthetic heart valves. Ann Thorac Surg. 1987;44(4):430-445. https://doi.org/10.1016/S0003-4975(10)63816-7
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165-3241. https://doi.org/10.1093/EURHEARTJ/EHY340
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/CHEST.11-2298
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336(21):1506-1511. https://doi.org/10.1056/NEJM199705223362107
- Tinker JH, Tarhan S. Discontinuing Anticoagulant Therapy in Surgical Patients With Cardiac Valve Prostheses: Observations in 180 Operations. JAMA. 1978;239(8):738-739. https://doi.org/10.1001/JAMA.1978.03280350062016
- Lankiewicz MW, Hays J, Friedman KD, Tinkoff G, Blatt PM. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost. 2006;4(5):967-970. https://doi.org/10.1111/J.1538-7836.2006.01815.X
- Renda G, Ricci F, Giugliano RP, de Caterina R. Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation and Valvular Heart Disease. J Am Coll Cardiol. 2017;69(11):1363-1371. https://doi.org/10.1016/J.JACC.2016.12.038
- Hammerstingl C, Tripp C, Schmidt H, von der Recke G, Omran H. Periprocedural Bridging Therapy with Low-Molecular-Weight Heparin in Chronically Anticoagulated Patients with Prosthetic Mechanical Heart Valves: Experience in 116 Patients from the Prospective BRAVE Registry. Published online 2007.
- Hjellström L, Labaf A. Prophylactic doses of low-molecular weight heparin as periprocedural bridging therapy in mechanical heart valve patients. Thromb Res. 2018;163:180-184. https://doi.org/10.1016/J.THROMRES.2017.09.023
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067. https://doi.org/10.1016/J.JACC.2017.09.1085
- Tsu L v., Dienes JE, Dager WE. Vitamin K dosing to reverse warfarin based on INR, route of administration, and home warfarin dose in the acute/critical care setting. Ann Pharmacother. 2012;46(12):1617-1626. https://doi.org/10.1345/APH.1R497
- Pernod G, Godiér A, Gozalo C, Tremey B, Sié P. French clinical practice guidelines on the management of patients on vitamin K antagonists in at-risk situations (overdose, risk of bleeding, and active bleeding). Thromb Res. 2010;126(3). https://doi.org/10.1016/J.THROMRES.2010.06.017
- Weibert RT, Le DT, Kayser SR, Rapaport SI. Correction of excessive anticoagulation with low-dose oral vitamin K1. Ann Intern Med. 1997;126(12):959-962. https://doi.org/10.7326/0003-4819-126-12-199706150-00005
- CV P, PA R, J E, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373(6). https://doi.org/10.1056/NEJMOA1502000
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015;373(25):2413-2424. https://doi.org/10.1056/NEJMOA1510991
- Connolly SJ, Milling TJ, Eikelboom JW, et al. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2016;375(12):1131-1141. https://doi.org/10.1056/NEJMOA1607887
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/NEJMOA1814051
- Dangas GD, Weitz JI, Giustino G, Makkar R, Mehran R. Prosthetic Heart Valve Thrombosis. J Am Coll Cardiol. 2016;68(24):2670-2689. https://doi.org/10.1016/J.JACC.2016.09.958
- Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389(10087):2383-2392. https://doi.org/10.1016/S0140-6736(17)30757-2
- Sondergaard L, de Backer O, Kofoed KF, et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur Heart J. 2017;38(28):2201-2207. https://doi.org/10.1093/EURHEARTJ/EHX369
- Montorsi P, de Bernardi F, Muratori M, Cavoretto D, Pepi M. Role of cine-fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol. 2000;85(1):58-64. https://doi.org/10.1016/S0002-9149(99)00607-4
- Muratori M, Montorsi P, Teruzzi G, et al. Feasibility and diagnostic accuracy of quantitative assessment of mechanical prostheses leaflet motion by transthoracic and transesophageal echocardiography in suspected prosthetic valve dysfunction. Am J Cardiol. 2006;97(1):94-100. https://doi.org/10.1016/J.AMJCARD.2005.07.112
- Cianciulli TF, Lax JA, Beck MA, et al. Cinefluoroscopic assessment of mechanical disc prostheses: its value as a complementary method to echocardiography. J Heart Valve Dis. 2005;14(5):664-673. Accessed June 1, 2022. https://europepmc.org/article/med/16245506
- Symersky P, Budde RPJ, de Mol BAJM, Prokop M. Comparison of multidetector-row computed tomography to echocardiography and fluoroscopy for evaluation of patients with mechanical prosthetic valve obstruction. Am J Cardiol. 2009;104(8):1128-1134. https://doi.org/10.1016/J.AMJCARD.2009.05.061
- Bapat V, Attia R, Redwood S, et al. Use of transcatheter heart valves for a valve-in-valve implantation in patients with degenerated aortic bioprosthesis: technical considerations and results. J Thorac Cardiovasc Surg. 2012;144(6):1372-1380. https://doi.org/10.1016/J.JTCVS.2012.07.104
- Gündüz S, Özkan M, Kalçik M, et al. Sixty-Four-Section Cardiac Computed Tomography in Mechanical Prosthetic Heart Valve Dysfunction: Thrombus or Pannus. Circ Cardiovasc Imaging. 2015;8(12). https://doi.org/10.1161/CIRCIMAGING.115.003246
- Suh YJ, Lee S, Im DJ, et al. Added value of cardiac computed tomography for evaluation of mechanical aortic valve: Emphasis on evaluation of pannus with surgical findings as standard reference. Int J Cardiol. 2016;214:454-460. https://doi.org/10.1016/J.IJCARD.2016.04.011
- Barbetseas J, Nagueh SF, Pitsavos C, Toutouzas PK, Quiñones MA, Zoghbi WA. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J Am Coll Cardiol. 1998;32(5):1410-1417. https://doi.org/10.1016/S0735-1097(98)00385-4
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the. J Am Soc Echocardiogr. 2009;22(9):975-1014. https://doi.org/10.1016/J.ECHO.2009.07.013
- Özkan M, Gündüz S, Biteker M, et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging. 2013;6(2):206-216. https://doi.org/10.1016/J.JCMG.2012.10.016
- Suchá D, Symersky P, Tanis W, et al. Multimodality Imaging Assessment of Prosthetic Heart Valves. Circ Cardiovasc Imaging. 2015;8(9). https://doi.org/10.1161/CIRCIMAGING.115.003703
- Cáceres-Lóriga FM, Pérez-López H, Morlans-Hernández K, et al. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J Thromb Thrombolysis. 2006;21(2):185-190. https://doi.org/10.1007/S11239-006-4969-Y
- Özkan M, Çakal B, Karakoyun S, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation. 2013;128(5):532-540. https://doi.org/10.1161/CIRCULATIONAHA.113.001145
- Karthikeyan G, Senguttuvan NB, Joseph J, Devasenapathy N, Bahl VK, Airan B. Urgent surgery compared with fibrinolytic therapy for the treatment of left-sided prosthetic heart valve thrombosis: a systematic review and meta-analysis of observational studies. Eur Heart J. 2013;34(21):1557-1566. https://doi.org/10.1093/EURHEARTJ/EHS486
- Keuleers S, Herijgers P, Herregods MC, et al. Comparison of thrombolysis versus surgery as a first line therapy for prosthetic heart valve thrombosis. Am J Cardiol. 2011;107(2):275-279. https://doi.org/10.1016/J.AMJCARD.2010.09.013
- Nagy A, Nagy A, Dénes M, Lengyel M. Predictors of the Outcome of Thrombolytic Therapy in Prosthetic Mitral Valve Thrombosis: A Study of 62 Events Emiratis vs South Asian Young Patients With Acute Coronary Syndromes: Risk Factor Profiles, Presentations and In-Hospital Outcomes View project Predictors of the Outcome of Thrombolytic Therapy in Prosthetic Mitral Valve Thrombosis: A Study of 62 Events. Published online 2014. Accessed June 1, 2022. https://www.researchgate.net/publication/26322239
- Roudaut R, Lafitte S, Roudaut MF, et al. Management of prosthetic heart valve obstruction: fibrinolysis versus surgery. Early results and long-term follow-up in a single-centre study of 263 cases. Arch Cardiovasc Dis. 2009;102(4):269-277. https://doi.org/10.1016/J.ACVD.2009.01.007
- Tong AT, Roudaut R, Ozkan M, et al. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol. 2004;43(1):77-84. https://doi.org/10.1016/J.JACC.2003.08.028
- Bade AS, Shaikh SSA, Khemani H, Singh G, Bansal NO. Thrombolysis Is an Effective and Safe Therapy in Stuck Mitral Valves With Delayed Presentation as Well as Hemodynamically Unstable Patients: A Single Centre Study. Cardiol Res. 2018;9(3):161-164. https://doi.org/10.14740/CR708W
- Pragt H, van Melle JP, Javadikasgari H, et al. Mechanical valves in the pulmonary position: An international retrospective analysis. J Thorac Cardiovasc Surg. 2017;154(4):1371-1378.e1. https://doi.org/10.1016/J.JTCVS.2017.04.072
- Taherkhani M, Hashemi SR, Hekmat M, Safi M, Taherkhani A, Movahed MR. Thrombolytic Therapy for Right-Sided Mechanical Pulmonic and Tricuspid Valves: The Largest Survival Analysis to Date. Tex Heart Inst J. 2015;42(6):543-547. https://doi.org/10.14503/THIJ-14-4659
- Özkan M, Gündüz S, Gürsoy OM, et al. Ultraslow thrombolytic therapy: A novel strategy in the management of PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE: The Ultra-slow PROMETEE trial. Am Heart J. 2015;170(2):409-418.e1. https://doi.org/10.1016/J.AHJ.2015.04.025
- Puri R, Auffret V, Rodés-Cabau J. Bioprosthetic Valve Thrombosis. J Am Coll Cardiol. 2017;69(17):2193-2211. https://doi.org/10.1016/J.JACC.2017.02.051
- Puvimanasinghe JPA, Steyerberg EW, Takkenberg JJM, et al. Prognosis after aortic valve replacement with a bioprosthesis: predictions based on meta-analysis and microsimulation. Circulation. 2001;103(11):1535-1541. https://doi.org/10.1161/01.CIR.103.11.1535
- Jander N, Kienzle RP, Kayser G, Neumann FJ, Gohlke-Baerwolf C, Minners J. Usefulness of phenprocoumon for the treatment of obstructing thrombus in bioprostheses in the aortic valve position. Am J Cardiol. 2012;109(2):257-262. https://doi.org/10.1016/J.AMJCARD.2011.08.038
- Butnaru A, Shaheen J, Tzivoni D, Tauber R, Bitran D, Silberman S. Diagnosis and treatment of early bioprosthetic malfunction in the mitral valve position due to thrombus formation. Am J Cardiol. 2013;112(9):1439-1444. https://doi.org/10.1016/J.AMJCARD.2013.06.014
- Pislaru S v., Hussain I, Pellikka PA, et al. Misconceptions, diagnostic challenges and treatment opportunities in bioprosthetic valve thrombosis: lessons from a case series. Eur J Cardiothorac Surg. 2015;47(4):725-732. https://doi.org/10.1093/EJCTS/EZU201
- de Marchena E, Mesa J, Pomenti S, et al. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8(5):728-739. https://doi.org/10.1016/J.JCIN.2015.03.005
- Petrescu I, Egbe AC, Ionescu F, et al. Long-Term Outcomes of Anticoagulation for Bioprosthetic Valve Thrombosis. J Am Coll Cardiol. 2020;75(8):857-866. https://doi.org/10.1016/J.JACC.2019.12.037
- Sellers SL, Turner CT, Sathananthan J, et al. Transcatheter Aortic Heart Valves: Histological Analysis Providing Insight to Leaflet Thickening and Structural Valve Degeneration. JACC Cardiovasc Imaging. 2019;12(1):135-145. https://doi.org/10.1016/J.JCMG.2018.06.028
- Latib A, Naganuma T, Abdel-Wahab M, et al. Treatment and clinical outcomes of transcatheter heart valve thrombosis. Circ Cardiovasc Interv. 2015;8(4). https://doi.org/10.1161/CIRCINTERVENTIONS.114.001779
- Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(6):589-590. https://doi.org/10.1093/EHJCI/JEW025
- Leontyev S, Borger MA, Davierwala P, et al. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg. 2011;91(4):1120-1126. https://doi.org/10.1016/J.ATHORACSUR.2010.12.053
- Kaneko T, Vassileva CM, Englum B, et al. Contemporary Outcomes of Repeat Aortic Valve Replacement: A Benchmark for Transcatheter Valve-in-Valve Procedures. Ann Thorac Surg. 2015;100(4):1298-1304. https://doi.org/10.1016/J.ATHORACSUR.2015.04.062
- Jaussaud N, Gariboldi V, Grisoli D, et al. Risk of reoperation for mitral bioprosthesis dysfunction. J Heart Valve Dis. 2012;21(1):56-60. Accessed June 4, 2022. https://europepmc.org/article/med/22474743
- Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312(2):162-170. https://doi.org/10.1001/JAMA.2014.7246
- Ye J, Cheung A, Yamashita M, et al. Transcatheter Aortic and Mitral Valve-in-Valve Implantation for Failed Surgical Bioprosthetic Valves: An 8-Year Single-Center Experience. JACC Cardiovasc Interv. 2015;8(13):1735-1744. https://doi.org/10.1016/J.JCIN.2015.08.012
- Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. Journal of the American Society of Echocardiography. 2019;32(4):431-475. https://doi.org/10.1016/J.ECHO.2019.01.003
- Hascoet S, Smolka G, Bagate F, et al. Multimodality imaging guidance for percutaneous paravalvular leak closure: Insights from the multi-centre FFPP register. Arch Cardiovasc Dis. 2018;111(6-7):421-431. https://doi.org/10.1016/J.ACVD.2018.05.001
- García-Fernández MA, Cortés M, García-Robles JA, Gomez de Diego JJ, Perez-David E, García E. Utility of real-time three-dimensional transesophageal echocardiography in evaluating the success of percutaneous transcatheter closure of mitral paravalvular leaks. J Am Soc Echocardiogr. 2010;23(1):26-32. https://doi.org/10.1016/J.ECHO.2009.09.028
- Nombela-Franco L, Ribeiro HB, Urena M, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol. 2014;63(24):2643-2658. https://doi.org/10.1016/J.JACC.2014.02.573
- Ruiz CE, Hahn RT, Berrebi A, et al. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis: An Expert Statement. J Am Coll Cardiol. 2017;69(16):2067-2087. https://doi.org/10.1016/J.JACC.2017.02.038
- Akins CW, Bitondo JM, Hilgenberg AD, Vlahakes GJ, Madsen JC, MacGillivray TE. Early and late results of the surgical correction of cardiac prosthetic paravalvular leaks. J Heart Valve Dis. 2005;14(6):792-799; discussion 799. Accessed June 4, 2022. https://europepmc.org/article/med/16359061
- Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Percutaneous repair of paravalvular prosthetic regurgitation: acute and 30-day outcomes in 115 patients. Circ Cardiovasc Interv. 2011;4(4):314-321. https://doi.org/10.1161/CIRCINTERVENTIONS.110.960955
- Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgitation. J Am Coll Cardiol. 2011;58(21):2218-2224. https://doi.org/10.1016/J.JACC.2011.07.041
- Alkhouli M, Rihal CS, Zack CJ, et al. Transcatheter and Surgical Management of Mitral Paravalvular Leak: Long-Term Outcomes. JACC Cardiovasc Interv. 2017;10(19):1946-1956. https://doi.org/10.1016/J.JCIN.2017.07.046
- Alkhouli M, Zack CJ, Sarraf M, et al. Successful Percutaneous Mitral Paravalvular Leak Closure Is Associated With Improved Midterm Survival. Circ Cardiovasc Interv. 2017;10(12). https://doi.org/10.1161/CIRCINTERVENTIONS.117.005730
- Ruiz CE, Jelnin V, Kronzon I, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol. 2011;58(21):2210-2217. https://doi.org/10.1016/J.JACC.2011.03.074
- Phan K, Zhao DF, Wang N, Huo YR, Eusanio M di, Yan TD. Transcatheter valve-in-valve implantation versus reoperative conventional aortic valve replacement: a systematic review. J Thorac Dis. 2016;8(1):E83-E93. https://doi.org/10.3978/J.ISSN.2072-1439.2016.01.44
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121(16):1848-1857. https://doi.org/10.1161/CIRCULATIONAHA.109.924613
- Shah S, Alashi A, Pettersson GB, et al. Characteristics and longer-term outcomes of paravalvular leak after aortic and mitral valve surgery. J Thorac Cardiovasc Surg. 2019;157(5):1785-1792.e1. https://doi.org/10.1016/J.JTCVS.2018.08.096
- Bouhout I, Mazine A, Ghoneim A, et al. Long-term results after surgical treatment of paravalvular leak in the aortic and mitral position. J Thorac Cardiovasc Surg. 2016;151(5):1260-1266.e1. https://doi.org/10.1016/J.JTCVS.2015.11.046
- Karchmer AW, Chu VH, Otto CM. Prosthetic valve endocarditis: Epidemiology, clinical manifestations, and diagnosis.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633-638. https://doi.org/10.1086/313753
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol. 2017;69(3):325-344. https://doi.org/10.1016/J.JACC.2016.10.066
- Mgbojikwe N, Jones SR, Leucker TM, Brotman DJ. Infective endocarditis: Beyond the usual tests. Cleve Clin J Med. 2019;86(8):559-567. https://doi.org/10.3949/CCJM.86A.18120
- Mahmood M, Kendi AT, Ajmal S, et al. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J Nucl Cardiol. 2019;26(3):922-935. https://doi.org/10.1007/S12350-017-1092-8
- Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. https://doi.org/10.1001/JAMAINTERNMED.2014.2556
- Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. New England Journal of Medicine. 2019;380(5):415-424.
- Prendergast BD, Tornos P. Surgery for infective endocarditis: Who and when? Circulation. 2010;121(9):1141-1152. https://doi.org/10.1161/CIRCULATIONAHA.108.773598
- Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013;173(16):1495-1504.
- Fowler VG, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293(24):3012-3021.
- Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J. 2007;28(2):196-203.
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis–Prospective Cohort Study. Arch Intern Med. 2009;169(5):463-473.
- Spiliopoulos K, Haschemi A, Fink G, Kemkes BM. Infective endocarditis complicated by paravalvular abscess: A surgical challenge. An 11-year single center experience. Heart Surgery Forum. 2010;13(2). https://doi.org/10.1532/HSF98.20081141
- Head SJ, Mokhles MM, Osnabrugge RLJ, Bogers AJJC, Kappetein AP. Surgery in current therapy for infective endocarditis. Vasc Health Risk Manag. 2011;7:255.
- Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458-477. https://doi.org/10.1161/CIRCULATIONAHA.109.192665
- Lin AY, Saul T, Aldaas OM, et al. Early versus delayed lead extraction in patients with infected cardiovascular implantable electronic devices. JACC Clin Electrophysiol. 2021;7(6):755-763.
- Ghoreishi M, Foster N, Pasrija C, et al. Early operation in patients with mitral valve infective endocarditis and acute stroke is safe. Ann Thorac Surg. 2018;105(1):69-75.
- Orwat S, Diller GP, van Hagen IM, et al. Risk of Pregnancy in Moderate and Severe Aortic Stenosis: From the Multinational ROPAC Registry. J Am Coll Cardiol. 2016;68(16):1727-1737. https://doi.org/10.1016/J.JACC.2016.07.750
- Tzemos N, Silversides CK, Colman JM, et al. Late cardiac outcomes after pregnancy in women with congenital aortic stenosis. Am Heart J. 2009;157(3):474-480. https://doi.org/10.1016/J.AHJ.2008.10.020
- Arias F, J. Pineda. Aortic stenosis and pregnancy.
- Silversides CK, Colman JM, Sermer M, Farine D, Siu SC. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol. 2003;91(11):1386-1389. https://doi.org/10.1016/S0002-9149(03)00340-0
- Yap SC, Drenthen W, Pieper PG, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol. 2008;126(2):240-246. https://doi.org/10.1016/J.IJCARD.2007.03.134
- Sugishita Y, Ito I, Kubo T. Pregnancy in Cardiac Patients: Possible Influence of Volume Overload by Pregnancy on Pulmonary Circulation : PANEL DISCUSSION ON PUMP FAILURE OF THE HEART WITH COMPLICATIONS : 49th Annual Scientific Session of the Japanese Circulation Society. Jpn Circ J. 1986;50(4):376-383. https://doi.org/10.1253/JCJ.50.376
- de Santo LS, Romano G, della Corte A, et al. Mechanical Aortic Valve Replacement in Young Women Planning on Pregnancy: Maternal and Fetal Outcomes Under Low Oral Anticoagulation, a Pilot Observational Study on a Comprehensive Pre-Operative Counseling Protocol. J Am Coll Cardiol. 2012;59(12):1110-1115. https://doi.org/10.1016/J.JACC.2011.10.899
- Leśniak-Sobelga A, Tracz W, Kostkiewicz M, Podolec P, Pasowicz M. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases—maternal and fetal outcome. Int J Cardiol. 2004;94(1):15-23. https://doi.org/10.1016/J.IJCARD.2003.03.017
- Hameed A, Karaalp IS, Tummala PP, et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001;37(3):893-899. https://doi.org/10.1016/S0735-1097(00)01198-0
- Orwat S, Diller GP, van Hagen IM, et al. Risk of Pregnancy in Moderate and Severe Aortic Stenosis: From the Multinational ROPAC Registry. J Am Coll Cardiol. 2016;68(16):1727-1737. https://doi.org/10.1016/J.JACC.2016.07.750
- Vinayakumar D, Vinod G v., Madhavan S, Krishnan MN. Maternal and fetal outcomes in pregnant women undergoing balloon mitral valvotomy for rheumatic mitral stenosis. Indian Heart J. 2016;68(6):780-782. https://doi.org/10.1016/J.IHJ.2016.04.017
- Gulraze A, Kurdi W, Niaz FA, Fawzy ME. Mitral balloon valvuloplasty during pregnancy:The long term up to 17 years obstetric outcome and childhood development. Pak J Med Sci. 2014;30(1):86. https://doi.org/10.12669/PJMS.301.4305
- Salomé N, Dias CC, Ribeiro J, Gonçalves M, Fonseca C, Ribeiro VG. Balloon mitral valvuloplasty during pregnancy–our experience. Rev Port Cardiol. 2002;21(12):1437-1444. Accessed August 29, 2022. https://europepmc.org/article/med/12621917
- Weiss BM, von Segesser LK, Alon E, Seifert B, Turina MI. Outcome of cardiovascular surgery and pregnancy: A systematic review of the period 1984-1996. Am J Obstet Gynecol. 1998;179(6):1643-1653. https://doi.org/10.1016/S0002-9378(98)70039-0
- Becker RM. Intracardiac Surgery in Pregnant Women. Ann Thorac Surg. 1983;36(4):453-458. https://doi.org/10.1016/S0003-4975(10)60486-9
- Parry AJ, Westaby S. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 1996;61(6):1865-1869. https://doi.org/10.1016/0003-4975(96)00150-6
- Samiei N, Amirsardari M, Rezaei Y, et al. Echocardiographic Evaluation of Hemodynamic Changes in Left-Sided Heart Valves in Pregnant Women With Valvular Heart Disease. Am J Cardiol. 2016;118(7):1046-1052. https://doi.org/10.1016/J.AMJCARD.2016.07.005
- D’Souza R, Ostro J, Shah PS, et al. Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J. 2017;38(19):1509-1516. https://doi.org/10.1093/EURHEARTJ/EHX032
- Özkan M, Çakal B, Karakoyun S, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation. 2013;128(5):532-540. https://doi.org/10.1161/CIRCULATIONAHA.113.001145
- Tong AT, Roudaut R, Ozkan M, et al. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol. 2004;43(1):77-84. https://doi.org/10.1016/J.JACC.2003.08.028
- Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(6):589-590. https://doi.org/10.1093/EHJCI/JEW025
- Massel DR, Little SH. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2013;2013(7). https://doi.org/10.1002/14651858.CD003464.PUB2
- Garrett AD. Dabigatran vs. Warfarin in patients with mechanical heart valves. Drug Topics. 2013;369(DEC):1206-1220. https://doi.org/10.1056/NEJMOA1300615/SUPPL_FILE/NEJMOA1300615_DISCLOSURES.PDF
- Bajaj A, Pancholy S, Sethi A, Rathor P. Safety and feasibility of PCI in patients undergoing TAVR: A systematic review and meta-analysis. Heart Lung. 2017;46(2):92-99. https://doi.org/10.1016/J.HRTLNG.2016.12.003
- Chakravarty T, Sharma R, Abramowitz Y, et al. Outcomes in Patients With Transcatheter Aortic Valve Replacement and Left Main Stenting: The TAVR-LM Registry. J Am Coll Cardiol. 2016;67(8):951-960. https://doi.org/10.1016/J.JACC.2015.10.103
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629-638. https://doi.org/10.1016/S0140-6736(13)60141-5
- Thalji NM, Suri RM, Daly RC, et al. The prognostic impact of concomitant coronary artery bypass grafting during aortic valve surgery: implications for revascularization in the transcatheter era. J Thorac Cardiovasc Surg. 2015;149(2):451-460.e2. https://doi.org/10.1016/J.JTCVS.2014.08.073
- Faroux L, Guimaraes L, Wintzer-Wehekind J, et al. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74(3):362-372. https://doi.org/10.1016/J.JACC.2019.06.012
- Goel SS, Ige M, Tuzcu EM, et al. Severe aortic stenosis and coronary artery disease–implications for management in the transcatheter aortic valve replacement era: a comprehensive review. J Am Coll Cardiol. 2013;62(1):1-10. https://doi.org/10.1016/J.JACC.2013.01.096
- Sankaramangalam K, Banerjee K, Kandregula K, et al. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J Am Heart Assoc. 2017;6(10). https://doi.org/10.1161/JAHA.117.006092
- Millan-Iturbe O, Sawaya FJ, Lønborg J, et al. Coronary artery disease, revascularization, and clinical outcomes in transcatheter aortic valve replacement: Real-world results from the East Denmark Heart Registry. Catheter Cardiovasc Interv. 2018;92(4):818-826. https://doi.org/10.1002/CCD.27440
- Witberg G, Regev E, Chen S, et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10(14):1428-1435. https://doi.org/10.1016/J.JCIN.2017.04.035
- Chieffo A, Giustino G, Spagnolo P, et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2015;8(7). https://doi.org/10.1161/CIRCINTERVENTIONS.114.002025
- Rossi A, de Cecco CN, Kennon SRO, et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2017;11(5):338-346. https://doi.org/10.1016/J.JCCT.2017.06.001
- van den Boogert TPW, Vendrik J, Claessen BEPM, et al. CTCA for detection of significant coronary artery disease in routine TAVI work-up : A systematic review and meta-analysis. Neth Heart J. 2018;26(12):591-599. https://doi.org/10.1007/S12471-018-1149-6
- Pesarini G, Scarsini R, Zivelonghi C, et al. Functional Assessment of Coronary Artery Disease in Patients Undergoing Transcatheter Aortic Valve Implantation: Influence of Pressure Overload on the Evaluation of Lesions Severity. Circ Cardiovasc Interv. 2016;9(11). https://doi.org/10.1161/CIRCINTERVENTIONS.116.004088
- Ahmad Y, Götberg M, Cook C, et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc Interv. 2018;11(20):2019-2031. https://doi.org/10.1016/J.JCIN.2018.07.019
- Yamanaka F, Shishido K, Ochiai T, et al. Instantaneous Wave-Free Ratio for the Assessment of Intermediate Coronary Artery Stenosis in Patients With Severe Aortic Valve Stenosis: Comparison With Myocardial Perfusion Scintigraphy. JACC Cardiovasc Interv. 2018;11(20):2032-2040. https://doi.org/10.1016/J.JCIN.2018.07.027
- Graboys TB, Cohn PF. The prevalence of angina pectoris and abnormal coronary arteriograms in severe aortic valvular disease. Am Heart J. 1977;93(6):683-686. https://doi.org/10.1016/S0002-8703(77)80062-8
- Ramsdale DR, Bennett DH, Bray CL, Ward C, Beton DC, Faragher EB. Angina, coronary risk factors and coronary artery disease in patients with valvular disease. A prospective study. Eur Heart J. 1984;5(9):716-726. https://doi.org/10.1093/OXFORDJOURNALS.EURHEARTJ.A061732
- Dangas G, Khan S, Curry BH, Kini AS, Sharma SK. Angina pectoris in severe aortic stenosis. Cardiology. 1999;92(1):1-3. https://doi.org/10.1159/000006938
- Basta LL, Raines D, Najjar S, Kioschos JM. Clinical, haemodynamic, and coronary angiographic correlates of angina pectoris in patients with severe aortic valve disease. Br Heart J. 1975;37(2):150-157. https://doi.org/10.1136/HRT.37.2.150
- Thalji NM, Suri RM, Daly RC, et al. Assessment of coronary artery disease risk in 5463 patients undergoing cardiac surgery: when is preoperative coronary angiography necessary? J Thorac Cardiovasc Surg. 2013;146(5). https://doi.org/10.1016/J.JTCVS.2013.06.046
- Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38(1):46-51. https://doi.org/10.1016/0002-9149(76)90061-8
- Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350-1358. https://doi.org/10.1056/NEJM197906143002402
- Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95(3):252-258. https://doi.org/10.1136/HRT.2008.149088
- Gahl K, Sutton R, Pearson M, Caspari P, Lairet A, McDonald L. Mitral regurgitation in coronary heart disease. Br Heart J. 1977;39(1):13-18. https://doi.org/10.1136/HRT.39.1.13
- Enriquez-Sarano M, Klodas E, Garratt KN, Bailey KR, Tajik AJ, Holmes DR. Secular trends in coronary atherosclerosis–analysis in patients with valvular regurgitation. N Engl J Med. 1996;335(5):316-322. https://doi.org/10.1056/NEJM199608013350504
- Breisblatt WM, Cerqueira M, Francis CK, Plankey M, Zaret BL, Berger HJ. Left ventricular function in ischemic mitral regurgitation–a precatheterization assessment. Am Heart J. 1988;115(1 Pt 1):77-82. https://doi.org/10.1016/0002-8703(88)90520-0
- Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699. https://doi.org/10.1016/J.JACC.2009.11.013
- Opolski MP, Kim WK, Liebetrau C, et al. Diagnostic accuracy of computed tomography angiography for the detection of coronary artery disease in patients referred for transcatheter aortic valve implantation. Clin Res Cardiol. 2015;104(6):471-480. https://doi.org/10.1007/S00392-014-0806-Z
- Andreini D, Pontone G, Mushtaq S, et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am Heart J. 2014;168(3):332-339. https://doi.org/10.1016/J.AHJ.2014.04.022
- Chieffo A, Giustino G, Spagnolo P, et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2015;8(7). https://doi.org/10.1161/CIRCINTERVENTIONS.114.002025
- Matsumoto S, Yamada Y, Hashimoto M, et al. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur Radiol. 2017;27(5):1963-1970. https://doi.org/10.1007/S00330-016-4547-4
- Rossi A, de Cecco CN, Kennon SRO, et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2017;11(5):338-346. https://doi.org/10.1016/J.JCCT.2017.06.001
- Byrne JG, Leacche M, Vaughan DE, Zhao DX. Hybrid cardiovascular procedures. JACC Cardiovasc Interv. 2008;1(5):459-468. https://doi.org/10.1016/J.JCIN.2008.07.002
- Hamdan A, Wellnhofer E, Konen E, et al. Coronary CT angiography for the detection of coronary artery stenosis in patients referred for transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2015;9(1):31-41. https://doi.org/10.1016/J.JCCT.2014.11.008
- Pontone G, Andreini D, Bartorelli AL, et al. Feasibility and accuracy of a comprehensive multidetector computed tomography acquisition for patients referred for balloon-expandable transcatheter aortic valve implantation. Am Heart J. 2011;161(6):1106-1113. https://doi.org/10.1016/J.AHJ.2011.03.003
- Lund O, Nielsen TT, Pilegaard HK, Magnussen K, Knudsen MA. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 1990;100(3):327-337. https://doi.org/10.1016/S0022-5223(19)35524-2
- Beach JM, Mihaljevic T, Svensson LG, et al. Coronary artery disease and outcomes of aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2013;61(8):837-848. https://doi.org/10.1016/J.JACC.2012.10.049
- Ad N, Henry L, Hunt S, Holmes SD. Do we increase the operative risk by adding the Cox Maze III procedure to aortic valve replacement and coronary artery bypass surgery? J Thorac Cardiovasc Surg. 2012;143(4):936-944. https://doi.org/10.1016/J.JTCVS.2011.12.018
- Badhwar V, Rankin JS, Damiano RJ, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg. 2017;103(1):329-341. https://doi.org/10.1016/J.ATHORACSUR.2016.10.076
- Ad N, Holmes SD, Pritchard G, Shuman DJ. Association of operative risk with the outcome of concomitant Cox Maze procedure: a comparison of results across risk groups. J Thorac Cardiovasc Surg. 2014;148(6):3027-3033. https://doi.org/10.1016/J.JTCVS.2014.05.039
- Ad N, Holmes SD, Rongione AJ, et al. The long-term safety and efficacy of concomitant Cox maze procedures for atrial fibrillation in patients without mitral valve disease. J Thorac Cardiovasc Surg. 2019;157(4):1505-1514. https://doi.org/10.1016/J.JTCVS.2018.09.131
- Gillinov AM, Bakaeen F, McCarthy PM, et al. Surgery for paroxysmal atrial fibrillation in the setting of mitral valve disease: a role for pulmonary vein isolation? Ann Thorac Surg. 2006;81(1):19-28. https://doi.org/10.1016/J.ATHORACSUR.2005.04.060
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372(15):1399-1409. https://doi.org/10.1056/NEJMOA1500528
- Abreu Filho CAC, Lisboa LAF, Dallan LAO, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation. 2005;112(9 Suppl). https://doi.org/10.1161/CIRCULATIONAHA.104.526301
- Akpinar B, Guden M, Sagbas E, et al. Combined radiofrequency modified maze and mitral valve procedure through a port access approach: early and mid-term results. Eur J Cardiothorac Surg. 2003;24(2):223-230. https://doi.org/10.1016/S1010-7940(03)00258-6
- Chua YL, Schaff H v., Orszulak TA, Morris JJ. Outcome of mitral valve repair in patients with preoperative atrial fibrillation: Should the maze procedure be combined with mitral valvuloplasty? J Thorac Cardiovasc Surg. 1994;107(2):408-415. https://doi.org/10.1016/S0022-5223(12)70085-5
- Deneke T, Khargi K, Grewe PH, et al. Efficacy of an additional MAZE procedure using cooled-tip radiofrequency ablation in patients with chronic atrial fibrillation and mitral valve disease. A randomized, prospective trial. Eur Heart J. 2002;23(7):558-566. https://doi.org/10.1053/EUHJ.2001.2841
- Jessurun ER, van Hemel NM, Defauw JJ, de La Rivière AB. A randomized study of combining maze surgery for atrial fibrillation with mitral valve surgery. Journal of Cardiovascular Surgery. 2003;44(1):9.
- Ad N, Holmes SD, Lamont D, Shuman DJ. Left-Sided Surgical Ablation for Patients With Atrial Fibrillation Who Are Undergoing Concomitant Cardiac Surgical Procedures. Ann Thorac Surg. 2017;103(1):58-65. https://doi.org/10.1016/J.ATHORACSUR.2016.05.093
- Huffman MD, Karmali KN, Berendsen MA, et al. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database Syst Rev. 2016;2016(8). https://doi.org/10.1002/14651858.CD011814.PUB2
- Huffman MD, Malaisrie SC, Karmali KN. Concomitant Atrial Fibrillation Surgery for People Undergoing Cardiac Surgery. JAMA Cardiol. 2017;2(3):334-335. https://doi.org/10.1001/JAMACARDIO.2016.5404
- Friedman DJ, Piccini JP, Wang T, et al. Association Between Left Atrial Appendage Occlusion and Readmission for Thromboembolism Among Patients With Atrial Fibrillation Undergoing Concomitant Cardiac Surgery. JAMA. 2018;319(4):365-374. https://doi.org/10.1001/JAMA.2017.20125
- Yao X, Gersh BJ, Holmes DR, et al. Association of Surgical Left Atrial Appendage Occlusion With Subsequent Stroke and Mortality Among Patients Undergoing Cardiac Surgery. JAMA. 2018;319(20):2116-2126. https://doi.org/10.1001/JAMA.2018.6024
- Johnsrud DO, Melduni RM, Lahr B, Yao X, Greason KL, Noseworthy PA. Evaluation of anticoagulation use and subsequent stroke in patients with atrial fibrillation after empiric surgical left atrial appendage closure: A retrospective case-control study. Clin Cardiol. 2018;41(12):1578-1582. https://doi.org/10.1002/CLC.23066
- Abrich VA, Narichania AD, Love WT, Lanza LA, Shen WK, Sorajja D. Left atrial appendage exclusion during mitral valve surgery and stroke in atrial fibrillation. J Interv Card Electrophysiol. 2018;53(3):285-292. https://doi.org/10.1007/S10840-018-0458-4
- García-Fernández MÁ, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003;42(7):1253-1258. https://doi.org/10.1016/S0735-1097(03)00954-9
- Noseworthy PA, Yao X, Deshmukh AJ, et al. Patterns of Anticoagulation Use and Cardioembolic Risk After Catheter Ablation for Atrial Fibrillation. J Am Heart Assoc. 2015;4(11). https://doi.org/10.1161/JAHA.115.002597
- Eitel C, Koch J, Sommer P, et al. Novel oral anticoagulants in a real-world cohort of patients undergoing catheter ablation of atrial fibrillation. Europace. 2013;15(11):1587-1593. https://doi.org/10.1093/EUROPACE/EUT128
- Melduni RM, Schaff H v., Lee HC, et al. Impact of Left Atrial Appendage Closure During Cardiac Surgery on the Occurrence of Early Postoperative Atrial Fibrillation, Stroke, and Mortality: A Propensity Score-Matched Analysis of 10 633 Patients. Circulation. 2017;135(4):366-378. https://doi.org/10.1161/CIRCULATIONAHA.116.021952
- Rankin JS, Grau-Sepulveda M v., Ad N, et al. Associations Between Surgical Ablation and Operative Mortality After Mitral Valve Procedures. Ann Thorac Surg. 2018;105(6):1790-1796. https://doi.org/10.1016/J.ATHORACSUR.2017.12.035