TS. PHẠM HỮU VĂN
(…)
7.1.6. Bệnh cơ tim tâm sinh
Những tiến bộ trong phẫu thuật sửa chữa và điều trị y tế đã cải thiện tiên lượng lâu dài của trẻ em sinh ra mắc bệnh tim bẩm sinh (CHD). Hơn 90% hiện sống sót đến tuổi trưởng thành, 882 và do đó tỷ lệ mắc bệnh CHD ở người trưởng thành ngày càng tăng. [883] Với ít bệnh nhân tử vong do các biến cố chu phẫu và suy tim sớm hơn, SCD đã trở thành nguyên nhân gây tử vong hàng đầu ở người lớn mắc bệnh CHD đã được sửa chữa. [884] Sự kết hợp của các vết mổ phẫu thuật, sẹo cơ tim và các bất thường về giải phẫu còn sót lại hoặc mới tạo thành nền tảng cho VA (Hình 26).
Phân tầng nguy cơ SCD ở bệnh nhân CHD và không có VA dai dẳng được ghi nhận vẫn còn khó khăn do nhóm bệnh nhân hỗn hợp, không có RCT, và các nghiên cứu quan sát tương đối nhỏ. Ở những bệnh nhân có sinh lý hai thất và LV hệ thống, tiêu chuẩn chuẩn của LVEF ≤ 35% được sử dụng để lựa chọn bệnh nhân cấy ICD phòng ngừa tiên phát. [885,886] Đối với những bệnh nhân ngất không giải thích được, các triệu chứng rối loạn nhịp tim nghiêm trọng như đánh trống ngực hoặc tiền ngất và NSVT, nên xem xét PES. [887] Ở những bệnh nhân không có triệu chứng với tứ chứng Fallot (TOF) nhưng có các chỉ số khác về chất nền VA, đánh giá điện sinh lý có thể tinh chỉnh phân tầng nguy cơ. [888] Thời đại phẫu thuật và các kỹ thuật được sử dụng ảnh hưởng đến chất nền VA và sự xuất hiện của VA và cần được xem xét. Ví dụ, việc sử dụng miếng vá sửa chữa xuyên hình khuyên ở bệnh nhân TOF được phát hiện có liên quan đến nguy cơ mắc VA thấp hơn. [889] Lợi ích của điều trị ICD phòng ngừa tiên phát ở bệnh nhân có RV đơn độc hoặc hệ thống không có bệnh được xác lập rõ ràng và yêu cầu xem xét bệnh – và các yếu tố cụ thể của bệnh nhân. [890,891]
Ở những bệnh nhân CHD có VA dai dẳng hoặc sống sau CA, việc đánh giá toàn diện các yếu tố kích hoạt, gồm hình ảnh tim (đặc biệt là CMR) và đánh giá huyết động là rất quan trọng (Hình 26). [892] Nếu phát hiện các bất thường về giải phẫu đã có từ trước hoặc mới đòi hỏi cần can thiệp, kế hoạch điều trị nên xem xét việc lập bản đồ và cắt ngang chất nền VT trước khi phẫu thuật, vì khả năng tiếp cận có thể bị suy giảm sau phẫu thuật. [888,893,894]
Đánh giá và can thiệp trước phẫu thuật đặc biệt quan trọng ở những bệnh nhân TOF trải qua phẫu thuật định giá lại van phổi. Ở những bệnh nhân được chọn mắc CHD (bao gồm cả những người được sửa chữa vách liên nhĩ để chuyển vị trí của các động mạch lớn, phẫu thuật Fontan và dị thường Ebstein), đánh giá và điều trị SVT (như nhịp nhanh vào lại nhĩ hoặc nhịp nhanh vào lại AV) với dẫn truyền thất nhanh cũng nên được xem xét. [890,895,896]
Trong trường hợp không thể xác định có các yếu tố có thể đảo ngược được, cấy ICD để phòng ngừa SCD thứ phát được khuyến cáo. [349,350] Trong khi hệ thống ICD qua tĩnh mạch được sử dụng thường xuyên nhất và có ưu điểm chống nhịp tim nhanh và chống nhịp tim chậm, ICD dưới da có thể thay thế ở những bệnh nhân được chọn có đường tiếp cận tĩnh mạch hạn chế đến tâm thất hoặc có shunt trong tim.
Ở bệnh nhân CHD, MVT hầu hết xuất hiện do nhịp nhanh vào lại sử dụng các eo giải phẫu được bao bọc bởi van, vật liệu vá và vết mổ. Các nghiên cứu lập bản đồ và triệt phá sớm, đặc biệt ở bệnh nhân TOF, đã xác định các eo giải phẫu quan trọng của vị trí giải phẫu có thể tái tạo được. [897,898] Theo khái niệm này, các eo giải phẫu đó có thể được tái tạo và cắt ngang ở nhịp xoang trong quá trình triệt phá qua catheter bằng hệ thống lập bản đồ điện giải phẫu, đạt tỷ lệ thành công cấp tính là 80%. [888,899–901] Việc sử dụng block dẫn truyền qua các eo giải phẫu như một điểm cuối của thủ thuật, ngoài khả năng tạo ra VT, đã cải thiện hơn nữa kết quả triệt phá lâu dài. [888,899]
Theo đó, triệt phá qua ống thông được khuyến cáo đặc biệt ở những bệnh nhân TOF có SMVT tái phát. Do sự phức tạp của bệnh nhân CHD cũng như chất nền của VT, các thủ thuật đó nên được thực hiện tại các trung tâm có chuyên môn về triệt phá bệnh nhân CHD qua catheter. Triệt phá qua catheter có thể được xem xét thay cho cấy ICD ở những bệnh nhân TOF chọn lọc có SMVT và chức năng hai tâm thất được bảo tồn, cung cấp kết quả sau thủ thuật kết hợp của không thể tạo ra và block dẫn truyền qua eo giải phẫu có thể đạt được. [888,899]
Bảng khuyến cáo 38—Các khuyến cáo dành cho phân tầng nguy cơ và phòng ngừa đột tử tim tiên phát do bệnh tim bẩm sinh
| Các khuyến cáo | Classa | Levelb |
| Phân tầng nguy cơ và ngăn ngừa SCD tiên phát | ||
| Tất cả các bệnh nhân CHD | ||
| Ở người lớn mắc CHD có sinh lý hai tâm thất và tâm thất trái có biểu hiện suy tim có triệu chứng (NYHAII/III) và EF ≤35% mặc dù OMT ≥ 3 tháng, cấy ICD được chỉ định. [885,886] |
I |
C |
| Ở những bệnh nhân mắc CHD được cho là ngất do loạn nhịp và có rối loạn chức năng tâm thất ít nhất ở mức độ trung bình hoặc SMVT có thể được tạo ra trên PES, việc cấy ICD nên được xem xét. [887,889,902] |
IIa |
C |
| Ở những bệnh nhân có rối loạn chức năng tâm thất đơn hoặc RV hệ thống tiến triển với các yếu tố nguy cơ bổ sung c, cấy ICD có thể được xem xét. [890,891] |
IIb |
C |
| Tứ chứng Fallot | ||
| Ở những bệnh nhân sau khi sửa chữa TOF có triệu chứng rối loạn nhịp tim và NSVT, nên xem xét đánh giá điện sinh lý gồm PES. [889,903–905] |
IIa |
C |
| Ở những bệnh nhân sau khi sửa chữa TOF có triệu chứng rối loạn nhịp tim và PES dương tính, hoặc kết hợp các yếu tố nguy cơ khác d và PES dương tính, nên xem xét cấy ICD. |
IIa |
C |
| Ở những bệnh nhân sau khi sửa chữa TOF không có triệu chứng rối loạn nhịp tim, nhưng có sự kết hợp của các yếu tố nguy cơ khác, có thể xem xét đánh giá điện sinh lý, gồm PES. |
IIb |
C |
| Ở những bệnh nhân TOF đã được sửa chữa trải qua phẫu thuật hoặc thay van phổi qua da, việc lập bản đồ qua catheter trước phẫu thuật và cắt ngang các eo giải phẫu liên quan đến VT trước hoặc trong khi can thiệp có thể được xem xét. [894] |
IIb |
C |
AV: nhĩ thất; CHD: bệnh tim bẩm sinh; CMR: cộng hưởng từ tim; EF: phân suất tống máu; ICD: máy khử rung tim có thể cấy; LV: van trái; NSVT: nhịp nhanh thất đơn hình tạm thời; NYHA: Hiệp hội Tim mạch New York; OMT: điều trị nội tối ưu; PES: kích thích điện được lập trình; RV: thất phải; SCD: đột tử do tim; SMVT: nhịp nhanh thất đơn hình dai dẳng; TOF: tứ chứng Fallot; VT: nhịp nhanh thất.
a Class khuyến cáo.
b Mức độ bằng chứng.
c Dữ liệu rất ít và các yếu tố nguy cơ có thể đặc hiệu cho từng tổn thương, gồm VT tạm thời, NYHAII/III, hở van AV nặng và QRS rộng ≥ 140ms (chuyển vị đại động mạch).
d Các yếu tố nguy cơ khác gồm rối loạn chức năng RV hoặc LV vừa phải, CMR sẹo RV rộng, [906,907] thời gian QRS ≥180ms [886,908] và QRS phân đoạn nghiêm trọng. [909,910]
Bảng khuyến cáo 39—Các khuyến cáo dành cho phòng ngừa đột tử tim thứ phát và điều trị rối loạn nhịp thất trong bệnh ti bẩm sinh
| Các khuyến cáo | Classa | Levelb |
| Phân tầng nguy cơ và ngăn ngừa SCD tiên phát | ||
| Tất cả các bệnh nhân CHD | ||
| Ở những bệnh nhân CHD có VA dai dẳng, nên đánh giá các tổn thương còn sót lại hoặc các bất thường về cấu trúc mới. [892,893] | I | B |
| Ở những bệnh nhân CHD có VT không dung nạp / CA do VF được cứu thoát, cấy ICD được chỉ định sau khi loại trừ các nguyên nhân có thể đảo ngược. [349,350] |
I |
C |
| Ở những bệnh nhân CHD và các cú sốc SMVT hoặc ICD tái phát, có triệu chứng đối với SMVT không thể kiểm soát được bằng điều trị nội khoa hoặc lập trình lại ICD, việc triệt phá qua catheter được thực hiện tại các trung tâm chuyên khoa nên được xem xét. c [899–901] |
IIa |
C |
| Ở những bệnh nhân CHD được lựa chọn (gồm sửa chữa vách ngăn tâm nhĩ để chuyển vị trí các động mạch lớn, phẫu thuật Fontan và dị tật Ebstein) biểu hiện CA, nên xem xét đánh giá và điều trị SVT với dẫn truyền thất nhanh. [890,895] |
IIa |
C |
| Tứ chứng Fallot | ||
| Ở những bệnh nhân TOF đã được sửa chữa có biểu hiện SMVT hoặc điều trị ICD thích hợp, có triệu chứng tái phát cho SMVT, việc triệt phá qua catheter được thực hiện ở các trung tâm chuyên khoa được khuyến cáo. [899–901] |
I |
C |
| Ở những bệnh nhân TOF đã được sửa chữa với SMVT đang trải qua phẫu thuật hoặc thay van phổi qua da, nên xem xét lập bản đồ qua catheter trước phẫu thuật và cắt ngang các eo giải phẫu liên quan đến VT trước hoặc trong khi can thiệp. [888,893,894] |
IIa |
C |
| Ở những bệnh nhân TOF đã được sửa chữa với chức năng hai tâm thất được bảo tồn và SMVT có triệu chứng, triệt phá qua catheter hoặc triệt phá bằng phẫu thuật đồng thời được thực hiện tại các trung tâm chuyên khoa có thể được coi là một phương pháp thay thế cho liệu pháp ICD. [899,901] |
IIb |
C |
CA: ngừng tim; CHD: bệnh tim bẩm sinh; ICD: máy khử rung tim có thể cấy; SCD: đột tử do tim; SMVT: nhịp nhanh thất đơn hình dai dẳng; SVT: nhịp tim nhanh trên thất; TOF: tứ chứng Fallot; VA: rối loạn nhịp thất; VF: rung thất; VT: nhịp nhanh thất.
a Class khuyến cáo.
b Mức độ bằng chứng.
c Khuyến cáo cụ thể cho TOF (ClassI-B)
7.2. Bệnh điện học tiên phát
7.2.1. Rung thất nguyên phát
Chẩn đoán rung tâm thất nguyên phát (IVF) được thực hiện ở những người sống sót sau SCA, tốt nhất là có VF được ghi nhận, sau khi loại trừ các nguyên nhân về cấu trúc, bệnh kênh, chuyển hóa hoặc nhiễm độc. [135.222.911–913] Các xét nghiệm chẩn đoán gồm hóa học máu, ECG (bao gồm cả chuyển đạo cao), CT tim/chụp động mạch vành, đo từ xa/Holter, kiểm tra gắng sức, siêu âm tim, test chẹn kênh natri CMR (xem Phần 5.2.3, tình huống 3) . [135,222,911] Xét nghiệm di truyền đối với bệnh kênh và gen bệnh cơ tim có thể được xem xét với tỷ lệ đột biến là 3–17%. [249.914.915] Có thể xem xét đánh giá lâm sàng của các thành viên gia đình thế hệ thứ nhất, nhưng hiệu quả chẩn đoán thấp. Đặc biệt, tầm quan trọng của mẫu tái cực sớm (ERP) được phát hiện ở những người thân không có triệu chứng là không chắc chắn. [182.916]
Ở bệnh nhân IVF, cấy ICD làm giảm nguy cơ tử vong do rối loạn nhịp tim tới 68% so với amiodarone [352,917–921] (Hình 27). Các nghiên cứu quan sát với thời gian theo dõi trung bình 5–6 năm, 21,0–29,6% bệnh nhân IVF bị tái phát rối loạn nhịp tim, tỷ lệ sốc ICD hàng năm tương ứng là 3,6–5,7%, trong khi 4,5–17,5% gặp phải những cú sốc không phù hợp. [919–921] Isoproterenol, verapamil, hoặc quinidine đã được sử dụng để điều trị cấp thời tình trạng phóng điện ICD tái phát hoặc bão điện. [912,913,918,922–926] Trong một số nghiên cứu nhỏ, quinidine có hiệu quả cao trong việc giảm hoặc thậm chí ngăn ngừa khả năng gây rối loạn nhịp tim trong quá trình kích thích được lập trình. [923,924,926] Hơn nữa, một nghiên cứu hồi cứu ở 46 bệnh nhân cho thấy giảm số lần sốc ICD trung bình từ 7,5 trên mỗi bệnh nhân trong 2,9 năm xuống còn 0,9 cú sốc trên mỗi bệnh nhân trong 3,7 năm, với sự giảm các cơn bão tâm thất từ 36 xuống còn 3 sau khi bắt đầu dùng quinidine. [922] Ở những bệnh nhân có các đợt VF tái phát được kích hoạt do PVC tương tự không đáp ứng với điều trị nội khoa, triệt phá qua ống thông đã cho thấy thành công (Hình 28). [186.221.333.493.927–930] PVC phổ biến nhất có nguồn gốc từ hệ thống Purkinje và có thể bị loại bỏ với tỷ lệ thành công cấp thời cao là 87–100%. [186.221.333.493.927–930] Bản đồ giải phẫu điện chi tiết cũng có thể tiết lộ những thay đổi cấu trúc cục bộ (62,5% trong một nghiên cứu). [248]
Bảng khuyến cáo 40 — Khuyến cáo quản lý các bệnh nhân có rung thất nguyên phát
| Các khuyến cáo | Classa | Levelb |
| Đánh giá chẩn đoán | ||
| Người ta khuyến cáo VF nguyên phát được chẩn đoán ở người sống sót sau SCA tốt nhất là có tài liệu về VF, sau khi loại trừ nguyên nhân cấu trúc, bệnh lý kênh, chuyển hóa hoặc nhiễm độc. [222,911] |
I |
B |
| Xét nghiệm lâm sàng (tiền sử, ECG và ECG chuyển đạo trước tim cao, test gắng sức, siêu âm tim) của các thành viên gia đình thế hệ thứ nhất của bệnh nhân VF nguyên phát có thể được xem xét. [222,278,916] |
IIb |
B |
| Ở những bệnh nhân VF nguyên phát, xét nghiệm di truyền các gen liên quan đến bệnh lý kênh và bệnh cơ tim có thể được xem xét. [249,278,914,915] |
IIb |
B |
| Ngăn ngừa SCD thứ phát và điều trị VAs | ||
| Cấy ICD được khuyến cáo ở bệnh nhân
VF nguyên phát. [352,917–919] |
I | B |
| Truyền isoproterenol, verapamil hoặc quinidine để điều trị cấp tính cơn bão điện hoặc ICD phát sốc tái phát nên được xem xét trong VF nguyên phát. [912.913.918.922–924.926] |
IIa |
C |
| Quinidine nên được xem xét cho điều trị kéo dài ngăn chặn cơn bão điện hoặc ICD phóng điện tái phát trong VF nguyên phát. [923,924] |
IIa |
B |
| Việc triệt phá qua catheter do các nhà điện sinh lý có kinh nghiệm nên được xem xét ở bệnh nhân VF nguyên phát có các đợt tái phát
VF được kích hoạt bằng một PVC tương tự không đáp ứng với điều trị thuốc. [927–930] |
IIa |
C |
ECG: điện tâm đồ; ICD: máy khử rung tim có thể cấy; PVC: phức bộ thất sớm; SCA: ngừng tim đột ngột; SCD: đột tử do tim; VA: rối loạn nhịp thất; VF: rung tâm thất.
a Class khuyến cáo.
b Mức độ bằng chứng.
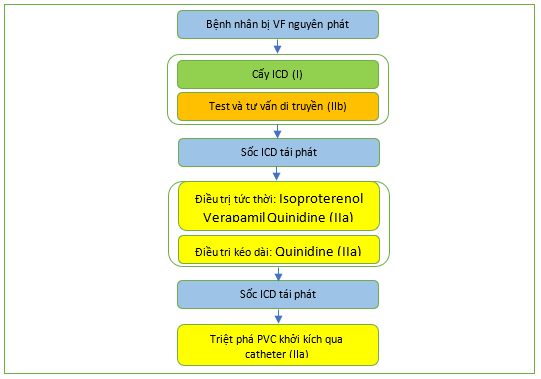
Hình 27. Thuật toán cho quản lý các bệnh nhân có rung thất nguyên phát, ICD: máy khử rung tim có thể cấy; PVC: phức bộ thất sớm.
7.2.2. Hội chứng QT dài (gồm hội chứng QT dài mắc phải)
LQTS được đặc trưng bởi khoảng QT kéo dài và VA chủ yếu được kích hoạt bởi hoạt hóa adrenergic. Độ tuổi trung bình tại thời điểm trình bày là 14 tuổi. Tỷ lệ SCD hàng năm ở những bệnh nhân không có triệu chứng với LQTS không được điều trị đã được ước tính là dưới 0,5%, [82] trong khi nó tăng lên khoảng 5% ở những người có tiền sử ngất. [931]
Các biến thể hiếm ở 17 gen [932] có liên quan đến LQTS. Tuy nhiên, mối quan hệ nhân quả đối với một số gen được xác định đã bị nghi ngờ. [166] Những gen không thể chối cãi là gen gây ra LQT1, LQT2
và LQTS3: KCNQ1, KCNH2 và SCN5A, tương ứng với các yếu tố kích hoạt gen đặc hiệu là gắng sức (LQTS1), cảm xúc căng thẳng (LQTS2) và ngủ (LQTS3). Sàng lọc di truyền xác định đột biến ở 75% trường hợp LQTS và ba gen chính chiếm 90% các trường hợp có kiểu gen dương tính. [178] Các loại phụ của LQTS có thể được nhóm lại như sau:
(1) LQTS nhiễm sắc thể thường trội (tỷ lệ mắc: 1 trên 2500) không có biểu hiện ngoài tim.
(2) LQTS nhiễm sắc thể thường có biểu hiện ngoài tim, bao gồm:
(a) Hội chứng Andersen–Tawil (LQT7), ngày càng được coi là thực thể của chính nó. [933,934]
(b) Hội chứng Timothy (LQT8), đặc trưng bằng QT kéo dài, dị tật ngón tay, dị tật tim, rối loạn phổ tự kỷ và rối loạn hình thái. [935]
(3) LQTS nhiễm sắc thể thường lặn (Hội chứng Jervell và Lange–Nielsen), kết hợp kéo dài QT quá mức với điếc bẩm sinh. [936]
Nhóm chuyên gia này đã xác nhận các tiêu chuẩn chẩn đoán được đề xuất trong phiên bản trước của hướng dẫn: QTc ≥ 480 ms hoặc điểm nguy cơ LQTS > 3 [937] (Bảng 10) để chẩn đoán lâm sàng (Hình
29 và 30). Khi có ngất hoặc CA do loạn nhịp tim, QTc ≥ 460 ms là đủ để xem xét chẩn đoán LQTS. Một thách thức đối với bác sĩ lâm sàng là thiết lập khoảng QT ở bệnh nhân có phức bộ QRS rộng (ví dụ như khi có nhịp thất hoặc khiếm khuyết dẫn truyền thất). Trong trạng thái này, một công thức
đã được đề xuất điều chỉnh QT theo thời lượng QRS. [938] Trong khi đo QT, bệnh nhân di chuyển nhanh từ tư thế nằm sang tư thế đứng có thể hữu ích cho chẩn đoán LQTS. [232,939] Phép thử epinephrine không được khuyến khích như một công cụ chẩn đoán thông thường vì khả năng tái tạo còn khiêm tốn. [137] Bệnh nhân được chẩn đoán lâm sàng LQTS được khuyến cáo trải qua tư vấn và xét nghiệm di truyền tại các trung tâm chuyên khoa để được quản lý theo kiểu gen cụ thể và cho phép xác định những người thân có nguy cơ. Những người thân có đột biến nhưng không kéo dài khoảng QT vẫn được chẩn đoán mắc LQTS, vì họ có nguy cơ bị VA, mặc dù ít gặp hơn so với những bệnh nhân có kiểu hình dương tính. [940]
Tất cả bệnh nhân LQTS đều nhận được lời tư vấn về cách tránh hạ kali máu, dùng thuốc kéo dài QT và các yếu tố kích hoạt đặc hiệu kiểu gen. [941–943] Thuốc chẹn beta cũng được khuyên dùng ở tất cả bệnh nhân LQTS. Thuốc chẹn beta không chọn lọc nadolol và propranolol có hiệu quả cao hơn trong việc giảm nguy cơ rối loạn nhịp tim. [940,944–946] Các thông số lâm sàng, điện tâm đồ và di truyền nên được xem xét để ước tính nguy cơ cá thể. [82] Gần đây, phân tầng nguy cơ dựa trên khoảng thời gian QT và kiểu gen đã được tích hợp trong máy tính LQTS (máy tính Rủi ro LQTS 1-2-3). [947]
Tiện ích của xét nghiệm di truyền được minh họa bằng nhu cầu tránh các nguy cơ đặc hiệu về kiểu gen và bằng cách sử dụng mexiletine như một phương pháp điều trị đặc hiệu kiểu gen cho LQT3, giúp làm giảm độ dài khoảng QT và số lượng các biến cố loạn nhịp tim. [948] Cần lưu ý rằng các đột biến khác nhau ở SCN5A cho thấy các phản ứng khác nhau với mexiletine. Ví dụ, các đột biến chọn lọc được xác định ở những bệnh nhân không đáp ứng với mexiletine cho thấy đặc điểm điện sinh lý đặc biệt khi nghiên cứu in vitro. [949,950] Ngoài ra, trong trường hợp đột biến gây ra hội chứng chồng chéo, mexiletine không tạo ra chênh lên của đoạn ST, trong khi flecainide đã được báo cáo làm chênh lên.
Do vai trò chưa chắc chắn của thuốc chẹn beta trong LQT3, không có dấu hiệu nào về việc nên dùng mexiletine như một liệu pháp độc lập hay kết hợp với thuốc chẹn beta. Xem xét rằng một số đột biến có thể không đáp ứng với mexiletine, nên thực hiện test uống để xác minh rằng QTc rút ngắn 40 mili giây trước khi kê đơn điều trị kéo dài. [948]
Những người sống sót sau CA có nguy cơ tái phát cao, ngay cả khi dùng thuốc chẹn beta (14% trong vòng 5 năm điều trị), ủng hộ việc sử dụng ICD ở những người sống sót sau CA. [952] Hơn nữa, cấy ICD được chỉ định khi bệnh nhân bị ngất và/hoặc VA mặc dù điều trị bằng thuốc tối ưu, vì các biến cố ngất có liên quan đến nguy cơ tăng CA. [953,954] Phụ nữ mắc LQTS, đặc biệt là LQT2, có nguy cơ rối loạn nhịp tim cao hơn cả khi mang thai và đặc biệt là năm đầu tiên sau sinh. [955] Những người mang đột biến thầm lặng có nguy cơ mắc các biến cố về tim thấp nhưng không đáng kể và việc sử dụng thuốc chẹn beta nên được xem xét ở nhóm bệnh nhân này. [956]
Bóc bỏ thần kinh giao cảm tim trái (LCSD) được khuyến cáo cho các bệnh nhân có triệu chứng mặc dù dùng thuốc chẹn beta khi ICD bị chống chỉ định hoặc bị từ chối, hoặc cho người mang ICD trải qua nhiều cú sốc khi đang dùng thuốc chẹn beta. Điều này được hỗ trợ bằng bằng chứng cho thấy LCSD, đặc biệt khi được thực hiện với kỹ thuật hỗ trợ bằng video, an toàn và hiệu quả, [957] được bệnh nhân dung nạp tốt [958] và không có tác động tiêu cực đến hoạt động tim mạch. [959] Tuy nhiên, do các biến chứng xảy ra và một nửa số bệnh nhân gặp phải các biến cố đột ngột sau thủ thuật, LCSD không phải là giải pháp thay thế cho ICD cho những bệnh nhân có nguy cơ cao. [960] Liệu pháp ICD dự phòng ngoài OMT có thể được xem xét ở những bệnh nhân LQTS không có triệu chứng được xác định có nguy cơ cao theo công cụ tính nguy cơ LQTS 1-2-3. [947] PES không hữu ích cho việc phân tầng nguy cơ trong LQTS. [961]
Hình 31 minh họa thuật toán quản lý bệnh nhân mắc hội chứng QT dài.
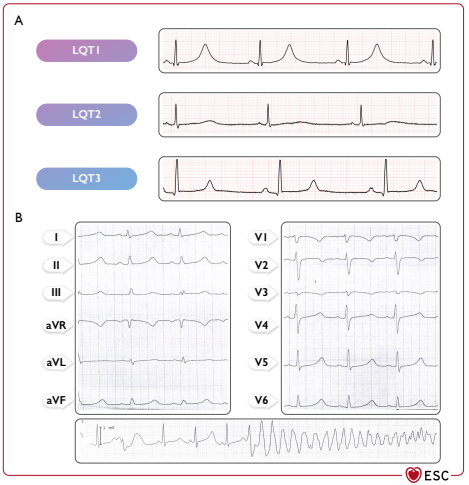
Hình 29 Điện tâm đồ hội chứng QT kéo dài và nhịp nhanh thất xoắn đỉnh. (A) Đặc điểm ECG trong ba type chính LQTS. (B) Ví dụ về xoắn đỉnh ở bệnh nhân nam có đột biến SCN5A (c.1238C.A, p. A413E). LQT: QT dài.
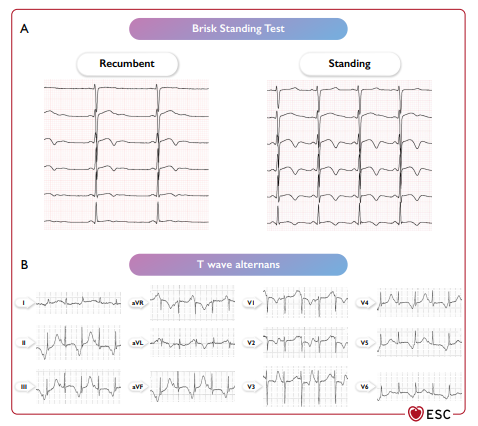
Hình 30 Thay đổi nhanh trên điện tâm đồ khi đứng và sự thay đổi sóng T ở bệnh nhân mắc hội chứng QT dài. (A) ECG thay đổi trong quá trình kiểm tra tư thế đứng nhanh ở bệnh nhân nam LQTS có đột biến KCNH2 (p.S818L), nhịp tim tăng có liên quan đến việc điều chỉnh khoảng QT ít hơn. (B) Sự thay đổi sóng T ở bệnh nhân nam có đột biến CACNA1C (p. G406R).
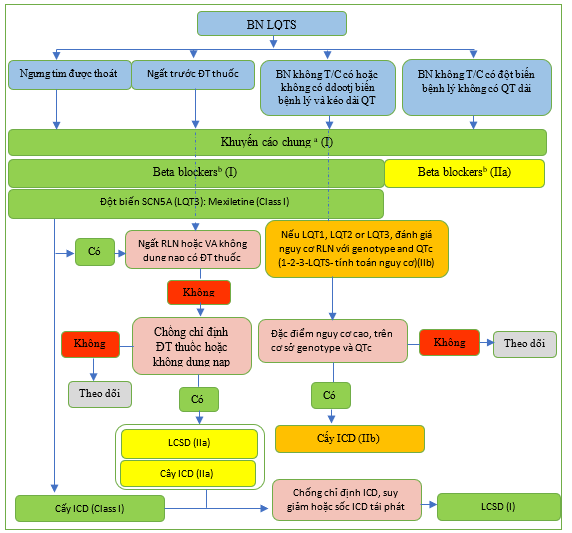
Hình 31 Sơ đồ quản lý bệnh nhân mắc hội chứng QT kéo dài. ICD, máy khử rung tim cấy ghép; LCSD: bóc bỏ thần kinh giao cảm tim trái; LQT, QT dài; N, Không; VA, rối loạn nhịp thất; Y: có. a Khuyến cáo chung: tránh dùng các thuốc kéó dài khoảng QT (http://www.creditmeds.org), điều chỉnh các bất thường về điện giải (hạ kali máu, hạ magie máu và hạ canxi máu), tránh các yếu tố kích thích đặc hiệu kiểu gen cho rồi loạn nhịp (bơi cường độ cao trong LQT1, tiếp xúc với tiếng ồn lớn trong LQT2). b Thuốc chẹn beta được ưu tiên: nadolol và propranolol.
(Còn nữa)
TÀI LIỆU THAM KHẢO
- Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J2000; 21: 2071–2078.
- Moss AJ, Hall WJ, Cannom DS, DaubertJP, Higgins SL, KleinH, etal. Improved survival with animplanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335: 1933–1940.
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, etal. Prophylactic implantation of a defibrillat or in patients with myocardial infarction and reduced ejection fraction. N Engl JMed2002;346: 877–883.
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999; 341: 1882–1890.
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, etal. Amiodarone or an implantable cardioverter – defibrillator for congestive heart failure. N Engl JMed 2005; 352: 225–237.
- Zabel M, Willems R, Lubinski A, Bauer A, Brugada J, Conen D, etal. Clinical effectiveness of primary prevention implantable cardioverter – defibrillators: results of the EU-CERT-ICD controlled multicentre cohort study. Eur Heart J 2020;41: 3437–3447.
- Schrage B, Uijl A, Benson L, Westermann D, Ståhlberg M, Stolfo D, et al. Association between use of primary – prevention implantable cardioverter defibrillators and mortality in patients with heart failure: aprospective propensity score-matched analysis from the Swedish Heart Failure Registry. Circulation 2019; 140:1530–1539.
- Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, etal. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375:1221–1230.
- Jukema JW, Timal RJ, Rotmans JI, Hensen LCR, Buiten MS, deBie MK, et al. Prophylacticuse of implantable cardioverter – defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation 2019;139: 2628–2638. 361. Sticherling C, Arendacka B, Svendsen JH, Wijers S, Friede T, Stockinger J, etal. Sex differences in outcomes of primary prevention implantable cardioverter defibrillator therapy: combined registry data from eleven European countries. Europace 2018;20: 963–970.
- Junttila MJ, Pelli A, Kenttä TV, Friede T, Willems R, Bergau L, etal. Appropriate shocks and mortality in patients with versus without diabetes with prophylactic implantable cardioverter defibrillators. Diabetes Care 2020;43: 196–200.
- Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S. Death with out prior appropriate implantable cardioverter – defibrillator therapy: acompeting risks tudy. Circulation 2008;117: 1918–1926.
- ClelandJ GF, Halliday BP, Prasad SK. Selecting patients with nonischemic dilated cardiomyopathy for ICDs: myocardial function, fibrosis, and what’s attached? J Am Coll Cardiol 2017; 70: 1228–1231.
- Younis A, Goldberger JJ, Kutyifa V, Zareba W, Polonsky B, Klein H, etal. Predicted benefit of an implantable cardioverter- defibrillator: the MADIT-ICD benefit score. Eur Heart J2021;42: 1676–1684.
- Knops RE, OldeNordkamp LRA, Delnoy P-PHM, Boersma LVA, Kuschyk J, El-Chami MF, etal. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020; 383: 526–536. 367.ClelandJGF, DaubertJ-C, Erdmann E, Freemantle N, Gras D,KappenbergerL, etal. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med2005; 352:1539–1549.
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140–2150.
- Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361: 1329–1338.
- Masri A, Altibi AM, Erqou S, Zmaili MA, Saleh A, Al-Adham R, etal. Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death: as ystematic review and meta-analysis. JACC Clin Electrophysiol 2019;5: 152–161.
- Garcia R, Combes N, Defaye P, Narayanan K, Guedon-Moreau L, Boveda S, etal. Wearable cardioverter-defibrillator in patients with a transient risk of sudden cardiac death: the WEARIT-France cohort study. Europace 2021; 23:73–81.
- Olgin JE, Pletcher MJ, Vittinghoff E, Wranicz J, Malik R, Morin DP, etal. Wearable cardioverter-defibrillator after myocardial infarction. N Engl J Med 2018;
379: 1205–1215.
- Scott PA, Silberbauer J, McDonagh TA, Murgatroyd FD. Impact of prolonged implantable cardioverter – defibrillator arrhythmia detection times on outcomes: a meta-analysis. Heart Rhythm 2014; 11:828–835. 374. Tan VH, Wilton SB, Kuriachan V, Sumner GL, Exner DV. Impact of programming strategies aimedat reducing non essential implantable cardioverter defibrillator therapies onmortality: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014; 7:164–170.
- Saeed M, HannaI, Robotis D, Styperek R, Polosajian L, Khan A, etal. Programming implantable cardioverter – defibrillators in patients with primaryprevention indication to prolong time to first shock: results from the PROVIDE study. J Cardiovasc Electrophysiol 2014;25: 52–59. 376. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, etal. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 2016;18: 159–183.
- Stiles MK, Fauchier L, Morillo CA, Wilkoff BL, ESC Scientific Document Group. 2019HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter – defibrillator programming and testing. Europace 2019;21: 1442–1443.
- Barsheshet A, Moss AJ, McNitt S, Jons C, Glikson M, Klein HU, etal. Long-term implications of cumulative right ventricular pacing among patients with an implantable cardioverter-defibrillator. Heart Rhythm2 011;8: 212–218.
- Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular back up pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002; 288: 3115–3123.
- Olshansky B, Day JD, Moore S, Gering L, Rosenbaum M, McGuireM, etal. Is dual chamber programming inferior to single-chamber programming in an implantable cardioverter-defibrillator? Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing With AVSH in ICDs) study. Circulation 2007;115: 9–16.
- Hindricks G, Küh lM, Dagres N. The implantable cardioverter defibrillator, conclusions on sudden cardiac death, and future perspective. ESC Cardio Med. 3rded. Oxford University Press; 2022, p2370–2376.
- Gasparini M, Proclemer A, Klersy C, Kloppe A, Ferrer JBM, Hersi A, etal. Effect of long-detection interval vs standard – detection interval for implantable cardioverter defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA 2013; 309: 1903–1911.
- Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367: 2275–2283.
- Wilkoff BL, Ousdigian KT, Sterns LD, Wang ZJ, Wilson RD, Morgan JM, etal. A comparison of empiric to physician-tailored programming of implantable cardioverter-defibrillators: results from the prospective randomized multicenter EMPIRIC trial. J am Coll Cardiol 2006; 48: 330–339.
- Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, etal. Strategicpro gramming of detection and therapy parameters in implantable cardioverter defibrillators reduces shocks inprimary prevention patients: results fromthe PREPARE (PrimaryPreventionParameters Evaluation) study. J AmColl Cardiol 2008;52: 541–550.
- Gilliam FR, Hayes DL, Boehmer JP, Day J, Heidenreich PA, Seth M, etal. Real world evaluation of dual-zone ICD and CRT-D programming compared to single-zone programming: the ALTITUDEREDUCES study. J Cardiovasc Electrophysiol 2011; 22:1023–1029.
- Hernandez-Ojeda J, Arbelo E, Borras R, Berne P, Tolosana JM, Gomez-Juanatey A, etal. Patients with Brugada syndrome and implanted cardioverter-defibrillators: long-term follow-up. J Am Coll Cardiol 2017;70: 1991–2002.
- Gold MR, Weiss R, Theuns DAMJ, Smith W, Leon A, Knight BP, etal. Use of adis crimination algorithm to reduce inappropriate shocks with a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2014;11: 1352–1358.
- Mesquita J, Cavaco D, Ferreira A, Lopes N, Santos PG, Carvalho MS, et al. Effectiveness of subcutaneous implantable cardioverter – defibrillators and determinants of inappropriate shock delivery. Int J Cardiol 2017;232: 176–180. 390. Gold MR, Lambiase PD, El-Chami MF, Knops RE, Aasbo JD, Bongiorni MG, etal. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with lowe jection fraction (UNTOUCHED) trial. Circulation 2021; 143:7–17.
- Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, etal. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREERxII) trial results. Circulation 2004;110: 2591–2596.
- Gulizia MM, Piraino L, Scherillo M, Puntrello C, Vasco C, Scianaro MC, etal. A randomized study to compareramp versus burst antitachycardia pacing therapies to treat fast ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators: the PITAGORA ICD trial. Circ Arrhythm Electrophysiol 2009; 2: 146–153.
- Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, etal. Long-term outcome after ICD and CRT implantation and influence of remotedevice follow up: the ALTITUDE survival study. Circulation 2010; 122: 2359–2367.
- Varma N, Piccini JP, Snell J, Fischer A, Dala lN, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemake rand defibrillator patients. J am Coll Cardiol 2015; 65: 2601–2610.
- Guédon-Moreau L, Kouakam C, Klug D, Marquié C, Brigadeau F, Boulé S, etal. Decreaseddelivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial. J Cardiovasc Electrophysiol 2014;25: 763–770.
- Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter – defibrillator lead and generator performance: the Lumos-T Safely Red Uce SRouTine Of ficeDevice Follow-Up (TRUST) trial. Circ Arrhythm Electrophysiol 2010;3: 428–436.
- Ploux S, Swerdlow CD, Strik M, Welte N, Klotz N, Ritter P, etal. Toward seradication of inappropriate therapies for ICD lead failure by combining comprehensive remote monitoring and lead noise alerts. J Cardiovasc Electrophysiol 2018;29: 1125–1134.
- Ellenbogen KA, Gunderson BD, Stromberg KD, Swerdlow CD. Performance of Lead Integrity Alert to assist in the clinical diagnosis of implantable cardioverter defibrillator lead failures: analysis of different implantable cardioverter defibrillator leads. Circ Arrhythm Electrophysiol 2013;6: 1169–1177.
- Swerdlow CD, Gunderson BD, Ousdigian KT, Abeyratne A, Sachanandani H, Ellenbogen KA. Downloadable software algorithm reduces inappropriate shocks caused by implantable cardioverter-defibrillator lead fractures: aprospective study. Circulation 2010;122: 1449–1455.
- Ruwald MH, Abu-Zeitone A, Jons C, Ruwald A-C, McNitt S, Kutyifa V, etal. Impact of carvedilol and metoprolol on inappropriate implantable cardioverter defibrillator therapy: the MADIT-CRT trial (Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy). J Am Coll Cardiol 2013; 62:1343–1350.
- Miyazaki S, Taniguchi H, Kusa S, Komatsu Y, Ichihara N, Takagi T, etal. Catheter ablation of atrial tachyarrhythmias causing inappropriate implantable cardioverter defibrillator shocks. Europace 2015; 17:289–294.
- Mainigi SK, Almuti K, Figueredo VM, Guttenplan NA, Aouthmany A, Smukler J, etal. Usefulness of radiofrequency ablation of supraventricular tachycardia to decrease inappropriate shocks from implantable cardioverter-defibrillators. AmJ Cardiol 2012;109: 231–237.
- Kosiuk J, Nedios S, Darma A, Rolf S, Richter S, Arya A, etal. Impact of single atrial fibrillation catheter ablation on implantable cardioverter defibrillator therapies in patients with ischaemic and non-ischaemic cardiomyopathies. Europace 2014;16: 1322–1326.
- Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, etal. Early rhythm control therapy in patients with atrial fibrillation. NEngl J Med 2020;383: 1305–1316.
- Gasparini M, Kloppe A, Lunati M, Anselme F, Landolina M, Martinez-Ferrer JB, etal. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter-defibrillator therapies and hospitalizations: atrioventricular junction ablation in CRT patients with AF. Eur J Heart Fail 2018; 20: 1472–1481.
- Gasparini M, Galimberti P. Ratecontro l: ablation and device therapy (ablateand pace). ESC Cardio Med. 3rded. Oxford University Press; 2022, p2159–2162.
- Kitamura T, Fukamizu S, Kawamura I, Hojo R, Aoyama Y, Komiyama K, et al. Long-term efficacy of catheter ablation for paroxysmal atrial fibrillation in patients with Brugada syndrome and an implantable cardioverter – defibrillator to prevent inappropriate shock therapy. Heart Rhythm 2016; 13:1455–1459.
- Magyar-Russe llG, Thombs BD, Cai JX, Baveja T, Kuhl EA, Singh PP, etal. The prevalence of anxiety and depression in adults with implantable cardioverter defibrillators: a systematic review. JP sychosom Res2011;71: 223–231.
- Tzeis S, Kolb C, Baumert J, Reents T, Zrenner B, Deisenhofer I, etal. Effect of depression on mortality in implantable cardioverter defibrillator recipients — findings from the prospective LICADstudy. Pacing Clin Electrophysiol 2011;34: 991–997.
- Andersen CM, Theuns DAMJ, Johansen JB, Pedersen SS. Anxiety, depression, ventricular arrhythmias and mortality in patients with an implantable cardioverter defibrillator: 7years’ follow-up of the MIDAS cohort. Gen Hosp Psychiatry 2020; 66: 154–160.
- Berg SK, Thygesen LC, Svendsen JH, Christensen AV, Zwisler A- D. Anxietypre dicts mortality in ICD patients: results from the cross-sectional national Copen Heart ICD survey with register follow-up. Pacing Clin Electrophysiol 2014; 37:1641–1650.
- Thylén I, Moser DK, Strömberg A, Dekker RA, Chung ML. Concerns about implantable cardioverter-defibrillator shocks mediate the relationship between actual shocks and psychological distress. Europace 2016; 18:828–835.
- Pedersen SS, van Domburg RT, Theuns DAMJ, Jordaens L, Erdman RAM. Concerns about the implantable cardioverter defibrillator: a determinant of anxiety and depressive symptoms independent of experienced shocks. AmHeart J 2005;149: 664–669.
- Frizelle DJ, Lewin B, Kaye G, Moniz-Cook ED. Development of a measure of the concerns held by people with implanted cardioverter defibrillators: the ICD C. Br J Health Psychol 2006;11: 293–301.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67: 361–370.
- Frydensberg VS, Johansen JB, Möller S, Riahi S, Wehberg S, Haarbo J, etal. Anxiety and depression symptoms in Danish patients with an implantable cardioverter defibrillator: prevalence and association with indication and sex up to2 years of follow-up (data from the national DEFIB-WOMEN study). Europace 2020;22: 1830–1840.
- Hoogwegt MT, Kupper N, Theuns DAMJ, Zijlstra WP, Jordaens L, Pedersen SS. Under treatment of anxiety and depression in patients with an implantable cardioverter-defibrillator: impact on health status. Health Psychol 2012;31: 745–753.
- Lane DA, Aguinaga L, Blomström-Lundqvist C, Boriani G, Dan G-A,Hills MT, etal. Cardiac tachyarrhythmias and patient values and preferences for their management: the European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2015; 17: 1747–1769.
- Dunbar SB, Dougherty CM, Sears SF, Carroll DL, Goldstein NE, Mark DB, etal. Educational andpsychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: a scientific statement from the American Heart Association. Circulation 2012;126: 2146–2172.
- Sears SF, SowellL DV, Kuhl EA, Kovacs AH, Serber ER, Handberg E, etal. The ICD shock and stress management program: a randomized trial of psychosocial treatment tooptimizequalityof life in ICDpatients. Pacing Clin Electrophysiol 2007; 30:858–864.
- Berg SK, Rasmussen TB, Herning M, Svendsen JH, Christensen AV, Thygesen LC. Cognitive behavioural therapy significantly reduces anxiety inpatientswith implanted cardioverter defibrillator compared with usual care: findings fromthe Screen-ICD randomized controlled trial. Eur J Prev Cardiol 2020;27: 258–268.
- Schulz SM, Ritter O, Zniva R, Nordbeck P, Wacker C, Jack M, etal. Efficacy of a web-based intervention for improving psychosocial well- being in patients with implantable cardioverter-defibrillators: the randomized controlled ICD- FORUM trial. Eur Heart J 2020; 41: 1203–1211.
- Vanden Broek KC, Tekle FB, Habibović M, Alings M, vanderVoort PH, Denollet J. Emotional distress, positive affect, and mortality in patients with an implantable cardioverter defibrillator. Int J Cardiol 2013; 165: 327–332.
- Hauptman PJ, Chibnall JT, Guild C, Armbrecht ES. Patient perceptions, physician communication, and the implantable cardioverter-defibrillator. JAMA Intern Med 2013; 173:571–577.
- Cikes M, Jakus N, Claggett B, Brugts JJ, Timmermans P, Pouleur A-C, etal. Cardiac implantable electronic devices with a defibrillator component and all-cause mortality in left ventricular assist device carriers: results from the PCHF-VAD registry. Eur J Heart Fail 2019; 21: 1129–1141.
- Galand V, Flécher E, Auffret V, Boulé S, Vincentelli A, Dambrin C, etal. Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. JACC Clin Electrophysiol 2018; 4: 1166–1175.
- Nakahara S, Chien C, Gelow J, Dalouk K, Henrikson CA, Mudd J, etal. Ventricular arrhythmias after left ventricular assist device. Circ Arrhythm Electrophysiol 2013; 6: 648–654. 428. Clerkin KJ, Topkara VK, Demmer RT, Dizon JM, Yuzefpolskaya M, Fried J A, et al. Implantable cardioverter-defibrillators in patients with a continuous-flow left ventricular assist device: a nanalysis of the INTERMACS registry. JACC Heart Fail 2017; 5: 916–926. 429. Oz MC, Rose EA, Slater J, Kuiper JJ, Catanese KA, Levin HR. Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. J Am Coll Cardiol 1994;24: 1688–1691. 430. Potapov EV, Antonides C, Crespo-Leiro MG, Combes A, Färber G, Hannan MM, etal. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg 56:230–270.
- Makki N, Mesubi O, Steyers C, Olshansky B, Abraham WT. Meta-analysis of the relation of ventricular arrhythmias to all-cause mortality after implantation of a left ventricular assist device. Am J Cardiol 2015;1 16:1385–1390.
- Yoruk A, Sherazi S, Massey HT, Kutyifa V, McNitt S, Hallinan W, etal. Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm 2016;13: 1052–1056.
- Bedi M, Kormos R, Winowich S, McNamara DM, Mathier MA, Murali S. Ventricular arrhythmias during left ventricular assistdevicesupport. AmJ Cardiol 2007; 99: 1151–1153.
- Brenyo A, Rao M, Koneru S, Hallinan W, Shah S, Massey HT, etal. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J Cardiovasc Electrophysiol 2012; 23: 515–520.
- Vakil K, Kazmirczak F, Sathnur N, Adabag S, Cantillon DJ, Kiehl EL, etal. Implantable cardioverter – defibrillator use in patients with left ventricular assist devices: a systematic review and meta-analysis. JACC Heart Fail 2016; 4: 772–779.
- Refaat MM, Tanaka T, Kormos RL, McNamara D, Teuteberg J, Winowich S, etal. Survivalbenefit of implantable cardioverter-defibrillators in left ventricular assist device-supported heart failure patients. J Card Fail 2012;18: 140–145.
- Cantillon DJ, Tarakji KG, Kumbhani DJ, Smedira NG, Starling RC, Wilkoff BL. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter-defibrillator. Heart Rhythm 2010;7: 466–471.
- Joyce E, Starling RC. HFrEF other treatment: ventricular assist devices. ESC Cardio Med. 3rded. Oxford University Press; 2022, p1884–1889. 439. Younes A, Al-Kindi SG, Alajaji W, Mackall JA, Oliveira GH. Presence of implantable cardioverter-defibrillators and wait- list mortality of patients supported with left ventricular assist devices asbridge toheart transplantation. Int J Cardiol 2017; 231:211–215.
- Agrawa lS, Garg L, Nanda S, Sharma A, Bhatia N, Manda Y, etal. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices–ameta-analysis. Int J Cardiol 2016; 222: 379–384.
- Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, etal. European Heart Rhythm Association (EHRA) international consensus docu menton how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID)incol laborationwiththeEuropeanAssociationforCardio-ThoracicSurgery(EACTS). Europace2020;22: 515–549.
- Burri H, Starck C, Auricchio A, Biffi M, Burri M, D’Avila A, etal. EHRA expertcon sensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: endorsedby the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-AmericanHeart RhythmSociety (LAHRS). Europace 2021;23: 983–1008.
- Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, etal. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380: 1895–1905. 444. Atti V, Turagam MK, Garg J, Koerber S, Angirekula A, Gopinathannair R, et al. Subclavian and axillary vein access versus cephalicvein cutdown or cardiac implantable electronic device implantation: ameta-analysis. JACC Clin Electrophysiol 2020;6: 661–671.
- BenzAP, Vamos M, Erath JW, Hohnloser SH. Cephalicvs. Subclavian lead implantation in cardiac implantable electronic devices: a systematic review and meta-analysis. Europace2019;21: 121–129.
446.ChanN-Y, Kwong N-P, Cheong A-P. Venous access and long-term pacemaker lead failure: comparing contrast-guided axillary vein puncture with subclavian puncture and cephalic cutdown. Europace 2017;19: 1193–1197.
- Defaye P, Boveda S, Klug D, Beganton F, Piot O, Narayanan K, etal. Dual-vs. single chamber defibrillators for primary prevention of sudden cardiac death: long-term follow-up of the Défibrillateur Automatique Implantable-Prévention Primaire registry. Europace2017;19: 1478–1484.
- Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD. Dual-chamber implantable cardioverter – defibrillators electionisassociated within creased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J Am Coll Cardiol 2011;58: 1007–1013.
- Friedman PA, Bradley D, Koestler C, Slusser J, Hodge D, Bailey K, etal. Aprospective randomized trial of single- or dual-chamber implantable cardioverter defibrillators to minimize inappropriate shock risk in primary sudden cardiac death prevention. Europace 2014;16: 1460–1468.
450.Chen B-W, Liu Q, Wang X, Dang A-M. Are dual-chamber implantable cardioverter-defibrillators really better than single-chamber ones? A systematic review and meta-analysis. J Interv Card Electrophysiol 2014;39: 273–280.
- Epstein LM, Love CJ, Wilkoff BL, Chung MK, Hackler JW, Bongiorni MG, etal. Superiorvenacava defibrillator coils make transvenous lead extraction more challenging and riskier. J Am Coll Cardiol 2013;61: 987–989.
- Larsen JM, Hjortshøj SP, Nielsen JC, Johansen JB, Petersen HH, Haarbo J, etal. Single-coil and dual-coil defibrillator leads and association with clinical outcomes in a complete Danish nation wide ICD cohort. Heart Rhythm 2016;13: 706–712.
- Kumar KR, Mandleywala SN, Madias C, Weinstock J, Rowin EJ, Maron BJ, etal. Singlecoil implantable cardioverter defibrillator leads in patients with hypertrophic cardiomyopathy. Am J Cardiol 2020;125: 1896–1900.
- Friedman PA, Rasmussen MJ, Grice S, rusty J, Glikson M, Stanton MS. Defibrillation thresholds are increased by right-sided implantation of totally transvenous implantable cardioverter defibrillators. Pacing Clin Electrophysiol 1999;22: 1186–1192.
- Stoevelaar R, Brinkman- Stoppelenburg A, Bhagwandien RE, vanBruchem-Visser RL, Theuns DA, vanderHeide A, etal. The incidence and impact of implantable cardioverter defibrillator shocks in the last phaseof life: an integratedreview. Eur J Cardiovasc Nurs 2018;17: 477–485.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Padeletti L, Arnar DO, Boncinelli L, Brachman J, Camm JA, Daubert JC, etal. EHRA Expert Consensus Statement on the management of cardiovascular implantable electronic devices in patients nearing end of life or requesting withdrawal of therapy. Europace2010;12: 1480–1489.
- Stoevelaar R, Brinkman-Stoppelenburg A, vanDriel AG, Theuns DA, Bhagwandien RE, van Bruchem-Visser RL, etal. Trends in time in the management of the implantable cardioverter defibrillator in the last phase of life: are trospective study of medical records. Eur J Cardiovasc Nurs 2019; 18: 449–457.
- Kirkpatrick JN, Gottlieb M, Sehgal P, Patel R, Verdino RJ. Deactivation of implantable cardioverter defibrillators interminal illness and end of life care. Am J Cardiol 2012; 109: 91–94.
- Stevenson WG, Khan H, Sager P, Saxon LA, Middlekauff HR, Natterson PD, etal. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late aftermyocardial infarction. Circulation 1993; 88: 1647–1670.
- De Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, deJonge N, etal. Slow conduction in the infarcted human heart. “Zigzag” course of activation. Circulation 1993;88: 915–926.
- De Chillou C, Lacroix D, Klug D, Magnin-PoullI, Marquié C, Messier M, etal. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation 2002; 105: 726–731.
- Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation 2003; 108:704–710.
- Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy. J am Coll Cardiol 2004; 43:1834–1842.
- Miljoen H, State S, Dechillou C, Magninpoull I, Dotto P, And ronache M, et al. Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace 2005; 7: 516–524.
- Priori SG, Blomström – Lundqvist C, Mazzanti A, Blom N, Borggrefe M, CammJ, etal. 2015 ESC Guidelines for the management of patients with ventricularar rhythmias and the prevention of sudden cardiac death: The task force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for EuropeanPaediatric andCongenital Cardiology (AEPC). EurHeart J 2015;36: 2793–2867.
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, etal. 2017AHA/ACC/HRS Guideline for management of patients with ventricularar rhythmias and the prevention of sudden cardiac death: executive summary. J Am Coll Cardiol 2018;72: 1677–1749.
- Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, etal. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: executive summary. Heart Rhythm 2019;16: e373–e407.
- Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, etal. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004; 110:3 760–3765.
- Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, RaittMH, et al. Prognostic importanceof defibrillator shocks in patients with heart failure. N Engl J Med 2008;359: 1009–1017.
- Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin J-F, etal. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl JMed 2016; 375:111–121.
- Piccini JP, Berger JS, O’Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J2009;30: 1245–1253.
- Palaniswamy C, Kolte D, Harikrishnan P, Khera S, Aronow WS, Mujib M, etal. Catheter ablation of post infarction ventricular tachycardia: ten – year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm 2014;11: 2056–2063.
- Caceres J, Jazayeri M, McKinnie J, Avitall B, Denker ST, Tchou P, etal. Sustained bundle branch reentry as amechanismof clinical tachycardia. Circulation1989;79: 256–270.
- Blanck Z, Dhala A, Deshpande S, Sra J, Jazayeri M, Akhtar M. Bundle branch reentrant ventricular tachycardia: cumulative experience in 48 patients. J Cardiovasc Electrophysiol 1993;4: 253–262.
- ChenH, Shi L, Yang B, JuW, Zhang F, Yang G, etal. Electrophysiological characteristics of bundle branch reentry ventricular tachycardia in patients without structural heart disease. Circ Arrhythm Electrophysiol 2018; 11: e006049.
- Pathak RK, Fahed J, Santangeli P, Hyman MC, Liang JJ, Kubala M, etal. Long-term outcome of catheter ablation for treatment of bundle branch re-entrant tachycardia. JACC Clin Electrophysiol 2018;4: 331–338.
- Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, etal. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation 2008;118: 2773–2782.
- DellaBella P, Baratto F, TsiachrisD, Trevisi N, Vergara P, Bisceglia C, et al. Managementof ventriculartachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation 2013; 127:1359–1368.
- Maury P, Baratto F, Zeppenfeld K, Klein G, Delacretaz E, Sacher F, et al. Radio-frequency ablation as primarymanagement of well-tolerated sustained monomorphic ventricular tachycardia inpatientswith structural heart disease andleftventricularejectionfractionover30%. Eur Heart J 2014;35: 1479–1485.
- Tung R, Vaseghi M, Franke lDS, Vergara P, DiBiase L, Nagashima K, etal. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group study. Heart Rhythm 2015;12: 1997–2007.
- Santangeli P, Zado ES, Supple GE, Haqqani HM, Garcia FC, Tschabrunn CM, etal. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol2015;8: 1413–1421.
- Marchlinski FE, Haffajee CI, Beshai JF, Dickfeld T-ML, Gonzalez MD, Hsia HH, etal. Long-term success of irrigated radiofrequency catheter ablation of sustained ventricular tachycardia. J Am Coll Cardiol 2016;67: 674–683.
- Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, etal. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl JMed2007;357: 2657–2665.
- Kuck K-H, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz E, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentrer and omised controlled trial. Lancet 2010;375: 31–40.
- Anter E, Kleber AG, Rottmann M, Leshem E, Barkagan M, Tschabrunn CM, etal. Infarct-related ventricular tachycardia. JACC Clin Electrophysiol 2018;4: 1033–1048. 487. Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000;101: 1288–1296.
- de Chillou C, Groben L, Magnin-Poull I, Andronache M, Abbas MM, Zhang N, etal. Localizing the critical isthmus of post infarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm 2014;11: 175–181.
- Jaïs P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, etal. Elimination of localab normal ventricular activities: a new endpoint for substrate modification in patients with scar-related ventricular rtachycardia. Circulation 2012;125: 2184–2196.
- Berruezo A, Fernandez-Armenta J. Lines, circles, channels, and clouds: looking for the best design for substrate-guided ablation of ventricular tachycardia. Europace 2014;16: 943–945.
- DiBiase L, Burkhardt JD, Lakkireddy D, Carbucicchio C, Mohanty S, Mohanty P, etal. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy. J Am Coll Cardiol 2015;66: 2872–2882.
- Berruezo A, Fernández – Armenta J, Andreu D, Penela D, Herczku C, Evertz R, etal. Scarde channeling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol 2015;8: 326–336.
- Haïssaguerre M, Shoda M, Jaïs P, Nogami A, Shah DC, Kautzner J, etal. Mappingand ablation of idiopathic ventricular fibrillation. Circulation 2002;106: 962–967.
- Shirai Y, Liang JJ, Santangeli P, Arkles JS, Schaller RD, Supple GE, etal. Comparison of the ventricular tachycardia circuit between patients with ischemic and nonischemic cardiomyopathies: detailed characterization by entrainment. Circ Arrhythm Electrophysiol 2019;12: e007249.
- Bhaskaran A, Tung R, Stevenson WG, Kumar S. Catheter ablation of VT in nonischaemic cardiomyopathies: endocardial, epicardial and intramural approaches. Heart Lung Circ 2019; 28: 84–101.
- Tung R, Raiman M, Liao H, Zhan X, Chung FP, Nagel R, etal. Simultaneous endocardial and epicardial delineation of 3D reentrant ventricular tachycardia. J am Coll Cardiol 2020;75: 884–897.
497.Dinov B, Fiedler L, Schönbauer R, Bollmann A, Rolf S, Piorkowski C, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014;129: 728–736.
- Proietti R, Essebag V, Beardsall J, Hache P, Pantano A, Wulffhart Z, et al. Substrate – guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long-term outcome. Europace 2015; 17:461–467.
- Ebert M, Richter S, Dinov B, Zeppenfeld K, Hindricks G. Evaluation and manage ment of ventricular tachycardia in patients with dilated cardiomyopathy. Heart Rhythm2019; 16:624–631.
- Proietti R, LichelliL, Lellouche N, Dhanjal T. The challenge of optimizing ablation lesions in catheter ablation of ventricular tachycardia. J Arrhythmia 2021;37: 140–147.
- Tokuda M, Sobieszczyk P, Eisenhauer AC, Kojodjojo P, Inada K, Koplan BA, etal. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhythm Electrophysiol 2011;4: 889–896.
- Kreidieh B, Rodríguez-Mañero M, Schurmann P, Ibarra – Cortez SH, Dave AS, Valderrábano M. Retrograde coronary venous ethanol infusion for ablation of refractory ventricular tachycardia. Circ Arrhythm Electrophysiol 2016;9: e004352.
- Nguyen DT, Tzou WS, Sandhu A, Gianni C, Anter E, Tung R, etal. Prospective multicenter experience with cooled radiofrequency ablation using high impedance irrigant to target deep myocardial substrate refractory to standard ablation. JACC Clin Electrophysiol 2018;4: 1176–1185.
- Stevenson WG, Tedrow UB, Reddy V, Abdel Wahab A, Dukkipati S, John RM, etal. Infusion needle radiofrequency ablation for treatment of refractory ventricularar rhythmias. J am Coll Cardiol 2019;73: 1413–1425.
- Igarashi M, Nogami A, Fukamizu S, Sekiguchi Y, Nitta J, Sakamoto N, etal. Acute and long-term results of bipolar radiofrequency catheter ablation of refractory ventricular arrhythmias of deep intramural origin. Heart Rhythm 2020;17: 1500–1507.
- Della Bella P, Peretto G, Paglino G, Bisceglia C, Radinovic A, Sala S, etal. Bipolar radiofrequency ablation for ventricular tachycardias originating from the interventricular septum: safety and efficacy in a pilot cohort study. Heart Rhythm 2020;17: 2111–2118.
- Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, etal. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 2017;377: 2325–2336.
- Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, etal. Phase I/ II trial of electrophysiology – guided noninvasive cardiac radio ablation for ventricular tachycardia. Circulation 2019;139: 313–321.
- Anter E, Hutchinson MD, Deo R, Haqqani HM, Callans DJ, Gerstenfeld EP, etal. Surgical ablation of refractory ventricular tachycardia in patients with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4: 494–500.
- Fernández-Armenta J, Berruezo A, Andreu D, Camara O, Silva E, Serra L, etal. Three-dimensional architecture of scar andconductingchannelsbasedonhigh resolution ce-CMR: insights for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2013;6: 528–537.
- Mahida S, Sacher F, Dubois R, Sermesan tM, Bogun F, Haïssaguerre M, etal. Cardiac imaging in patients with ventricular tachycardia. Circulation 2017; 136: 2491–2507.
- Andreu D, Penela D, Acosta J, Fernández-Armenta J, Perea RJ, Soto-Iglesias D, etal. Cardiac magnetic resonance–aided scarde channeling: influence on acute and long term outcomes. Heart Rhythm 2017; 14:1121–1128. 5
- Kuo L Liang JJ, Nazarian S, Marchlinski FE. Multimodality imaging to guide ventricular tachycardia ablation in patients with non – ischaemic cardiomyopathy. Arrhythm ElectrophysiolRev 2020;8: 255–264.
- Roca-LuqueI, Van Breukelen A, Alarcon F, Garre P, Tolosana JM, Borras R, etal. Ventricular scar channel entrances identified by new wide band cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. Europace 2020;22: 598–606.
- Betensky BP, Marchlinski FE. Outcomes of catheter ablation of ventricular tachycardia in the setting of structural heart disease. Curr Cardio lRep 2016; 18:68.
- Dukkipati SR, Koruth JS, Choudry S, Miller MA, Whang W, Reddy VY. Catheter ablation of ventricular tachycardia in structural heart disease. J am Coll Cardiol 2017; 70:2924–2941.
- Zeppenfeld K. Ventricular tachycardia ablation in nonischemic cardiomyopathy. JACC Clin Electrophysiol 2018;4: 1123–1140.
- Guandalini GS, LiangJ J, Marchlinski FE. Ventricular tachycardia ablation. JACC Clin Electrophysiol 2019;5: 1363–1383.
- Peich lP, Wichterle D, Pavlu L, Cihak R, Aldhoon B, Kautzner J. Complications of catheter ablation of ventricular tachycardia: a single-center experience. Circ ArrhythmElectrophysiol2014;7: 684–690.
- Katz DF, Turakhia MP, Sauer WH, Tzou WS, Heath RR, Zipse MM, etal. Safety of ventricular tachycardia ablation in clinical practice: findings from 9699 hospital discharge records. Circ Arrhythm Electrophysiol 2015;8: 362–370.
- Cheung JW, YeoI, Ip JE, Thomas G, Liu CF, Markowitz SM, etal. Outcomes, costs, and 30-day read missions after catheter ablation of myocardial infarct–associated ventricular tachycardia in the real world: nation wide read missions data base2010 to2015. Circ Arrhythm Electrophysiol 2018; 11: e006754.
- Hendriks AA, Akca F, Dabiri Abkenari L, Khan M, Bhagwandien R, Yap S-C, etal. Safety and clinical outcome of catheter ablation of ventricular arrhythmias using contact for cesensing: consecutive case series. J Cardiovasc Electrophysiol 2015;26: 1224–1229.
- Nogami A. Purkinje-related arrhythmias partI: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011;34: 624–650.
- Lerman BB. Mechanism, diagnosis, and treatment of outflow tract tachycardia. Nat Rev Cardiol 2015;12: 597–608.
- Kobayashi Y. Idiopathic ventricular premature contraction and ventricular tachycardia: distribution of the origin, diagnostic algorithm, andcatheter ablation. J Nippon Med Sch 2018;85: 87–94.
- Tada H, Ito S, Naito S, Kurosaki K, Kubota S, Sugiyasu A, etal. Idiopathic ventricular arrhythmia arising from the mitral annulus: adistinct subgroup of idiopathic ventricular arrhythmias. J am Coll Cardiol 2005; 45:877–886.
- Wasmer K, Köbe J, Dechering DG, Bittner A, Pott C, Mönnig G, etal. Ventricular arrhythmias from the mitral annulus: patient characteristics, electrophysiological findings, ablation, and prognosis. Heart Rhythm 2013;10: 783–788.
- Tada H, Tadokoro K, Ito S, Naito S, Hashimoto T, Kaseno K, etal. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007;4: 7–16.
- Yamada T, Doppalapudi H, Mc Elderry HT, Okada T, Murakami Y, Inden Y, etal. Electrocardiographic and electrophysiological characteristics in idiopathic ventricular arrhythmias originating from the papillary musclesin the left ventricle: relevance for catheter ablation. Circ Arrhythm Electrophysiol 2010;3: 324–331.
- Macias C, Nakamura K, Tung R, Boyle NG, Kalyanam S, Bradfield JS. Importance of delayed enhanced cardiac MRI in idiopathic RVOT-VT: differentiating mimics including early stage ARVC and cardiac sarcoidosis. J AtrFibrillation2014; 7:1097.
- Heeger C-H, Hayashi K, Kuck K-H, Ouyang F. Catheter ablation of idiopathic ventricular arrhythmias arising from the cardiac outflow tracts— recent insights and techniques for the successful treatment of common and challenging cases. Circ J 2016;80: 1073–1086.
- Pathak RK, Ariyarathna N, Garcia FC, Sanders P, Marchlinski FE. Catheter ablation of idiopathic ventricular arrhythmias. Heart Lung Circ 2019;28: 102–109.
- Yamada T, McElderry HT, Doppalapudi H, Murakami Y, Yoshida Y, Yoshida N, etal. Idiopathic ventricular arrhythmias originating from the aortic root prevalence, electrocardiographic andelectrophysiologic characteristics, and results of radiofrequency catheter ablation. Jam Coll Cardiol 2008;52: 139–147.
- Van Herendael H, Garcia F, Lin D, Riley M, Bala R, Cooper J, etal. Idiopathic right ventricular arrhythmias not arising from the outflow tract: prevalence, electrocardiographic characteristics, and outcome of catheter ablation. Heart Rhythm 2011;8: 511–518.
- Latchamsetty R, Yokokawa M, Morady F, Kim HM, Mathew S, Tilz R, et al. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol 2015;1: 116–123.
- Liu Y, Fang Z, Yang B, Kojodjojo P, Chen H, Ju W, etal. Catheter ablation of fascicular ventricular tachycardia: long-term clinical outcomes and mechanisms of recurrence. Circ Arrhythm Electrophysiol 2015;8: 1443–1451.
- Hayashi T, Liang JJ, Shirai Y, Kuo L, Muser D, Kubala M, etal. Trends insuccessful ablation sites and outcomes of ablation for idiopathic outflow tract ventricularar rhythmias. JACC Clin Electrophysiol 2020;6: 221–230.
- Farré J, Wellens HJ. Philippe Coumel: a founding father of modern arrhythmology. Europace 2004;6: 464–465.
- Neira V, Enriquez A, Simpson C, Baranchuk A. Update on long QT syndrome. J Cardiovasc Electrophysiol 2019;30: 3068–3078.
- Winbo A, Paterson DJ. The brain-heart connection in sympathetically triggered inherited arrhythmia syndromes. Heart Lung Circ 2020;29: 529–537.
- Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, etal. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 2004; 109: 1826–1833.
- Surman TL, Stuklis RG, Chan JC. Thoracoscopic sympathectomy for long QT syndrome. Literature review and case study. Heart Lung Circ 2019;28: 486–494.
- OrvinK, Eisen A, Goldenberg I, Gottlieb S, Kornowski R, Matetzky S, et al. Outcome of contemporary acute coronary syndrome complicated by ventricular tachyarrhythmias. Europace 2016;18: 219–226.
- Demire lF, Rasou lS, Elvan A, Ottervanger JP, Dambrink J- HE, Gosselink ATM, etal. Impactofout-of-hospital cardiac arrest due to ventricular fibrillation in patients with ST- elevation myocardial infarction admitted for primary percutaneous coronary intervention: Impact of ventricular fibrillation inSTEMIpatients. EurHeart J AcuteCardiovascCare2015;4: 16–23.
- Mehta RH, Starr AZ, Lopes RD, Hochman JS, Widimsky P, Piepe rKS, et al. Incidence of and outcomes associated with ventricula rtachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA2009; 301:1779–1789.
- Demidova MM, Carlson J, Erlinge D, Platonov PG. Predictors of ventricular fibrillation at reperfusion in patients with acute ST- elevation myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol 2015; 115:417–422.
- Cheng Y-J, LiZ-Y, YaoF-J,XuX-J, JiC-C, Chen X-M, etal. Early repolarization is associated with a significantly increased risk of ventricular arrhythmias and sudden cardiac death in patients with structural heart diseases. Heart Rhythm 2017;14: 1157–1164.
- Dumas F, Bougouin W, Geri G, Lamhaut L, Rosencher J, Pène F, etal. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-segment elevation pattern: insights from the PROCAT II registry. JACC CardiovascI nterv 2016;9: 1011–1018.
- Chatterjee S, Chaudhuri D, Vedanthan R, Fuster V, Ibanez B, Bangalore S, etal. Early intravenous beta-blockers in patients with acute coronary syndrome – a meta-analysis of randomized trials. Int J Cardiol2013;168: 915–921.
- Roolvink V, Ibáñez B, Ottervanger JP, Pizarro G, van Royen N, Mateos A, etal. Early intravenous beta-blockers in patients with ST- segment elevation myocardial infarction befor eprimary percutaneous coronary intervention. J am Coll Cardiol 2016; 67: 2705–2715.
- Piccini JP, Hranitzky PM, Kilaru R, Rouleau J-L, White HD, AylwardPE, et al. Relation of mortality to failure to prescribe beta blockers acutely in patients with sustained ventricular tachycardia and ventricular fibrillation following acute myocardial infarction (from the VALsartan In Acute myocardial iNfarcTion trial [VALIANT] Registry). Am J Cardiol 2008;102: 1427–1432.
- Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM. Treating electrical storm: sympathetic blockade versus advanced cardiac life support- guided therapy. Circulation 2000; 102:742–747.
- Bundgaard JS, Jacobsen PK, Grand J, Lindholm MG, Hassager C, Pehrson S, etal. Deep sedation as temporary bridge to definitive treatment of ventricular arrhythmia storm. Eur Heart J Acute Cardiovas cCare 2020;9: 657–664.
- Piccini JP, Schulte PJ, Pieper KS, Mehta RH, White HD, Vande Werf F, et al. Antiarrhythmic drug therapy for sustained ventricular arrhythmias complicating acute myocardial infarction. Crit Care Med 2011;39: 78–83.
- Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med 2002; 346:884–890.
- Echt DS, Liebson PR, MitchellL B, Peters RW, Obias – Manno D, Barker AH, etal. Mortality and morbidity inpatients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia SuppressionTrial. N Engl J Med 1991;324: 781–788.
- Baudry G, Sonneville R, Waintraub X, Lebreton G, Deguillard C, Mertens E, etal. Extracorporeal membrane oxygenation to suppor tlife – threatening drug- refractory electrical storm. Crit Care Med 2020; 48: e856–e863.
- Demidova MM, Smith JG, Höijer C-J, Holmqvist F, Erlinge D, Platonov PG. Prognostic impact of early ventricular fibrillation in patients with ST-elevation myocardial infarction treated with primary PCI. Eur Heart J Acute Cardiovasc Care 2012; 1:302–311.
- Podolecki T, Lenarczyk R, Kowalczyk J, Jedrzejczyk- Patej E, Chodor P, Mazurek M, et al. Prognostic significance of complex ventricular arrhythmias complicating ST-segment elevation myocardial infarction. Am J Cardiol 2018; 121:805–809.
- Bougouin W, Marijon E, Puymirat E, Defaye P, Celermajer DS, LeHeuzey J-Y, etal. Incidence of sudden cardiac death after ventricular fibrillation complicating acute myocardial infarction: a 5-year cause-of-death analysis of the FAST-MI2005 registry. Eur Heart J2014;35: 116–122.
- Liang JJ, Hodge DO, Mehta RA, Russo AM, Prasad A, Cha Y-M. Outcomes in patients with sustained ventricular tachyarrhythmias occurring within 48h of acute myocardial infarction: when is ICD appropriate? Europace 2014;16: 1759–1766.
- Ahn J-M, Lee KH, Yoo S-Y, Cho Y-R, Suh J, Shin E-S, etal. Prognosis of variant angina manifesting as aborted sudden cardiac death. J Am Coll Cardiol 2016; 68:137–145.
- Sueda S, Kohno H. Optimal medications and appropriate implantable cardioverter defibrillator shocks in aborted sudden cardiac death due to coronary spasm. In tern Med 2018;57: 1361–1369.
- Rodríguez -Mañero M, Oloriz T, le Polainde Waroux J-B, Burri H, Kreidieh B, de Asmundis C, etal. Long-term prognosis of patients with life – threatening ventricular arrhythmias induced by coronary artery spasm. Europace 2018;20: 851–858.
- Solomon SD, Zelenkofske S, McMurray JJV, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 2005; 352: 2581–2588.
- Busk M, Maeng M, Kristensen SD, Thuesen L, Krusell LR, Mortensen LS, et al. Timing, causes, and predictors of death after three years’ follow-up in the Danish Multicenter Randomized Study of Fibrinolysis versus Primary Angioplasty in Acute Myocardial Infarction (DANAMI-2) trial. Am J Cardiol 2009;104: 210–215.
- StJohn Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moyé LA, Dagenais GR, etal. Quantitative two – dimensional echocardiographic measurement sare major predictors of adverse cardiovascular event safter acute myocardial infarction. The protective effects of captopril. Circulation 1994; 89:68–75.
- Søholm H, Lønborg J, Andersen MJ, Vejlstrup N, Engstrøm T, Møller JE, et al. Repeated echocardiography after first ever ST- segment elevation myocardial in farction treated with primary percutaneous coronary intervention– is it necessary? Eur Heart J Acute Cardiovasc Care 2015;4: 528–536.
- Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361: 1427–1436.
- Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 2004;351: 2481–2488.
- Exner DV, Kavanagh KM, Slawnych MP, MitchellL B, Ramadan D, Aggarwal SG, etal. Noninvasive risk assessment early after amyocardial infarction the REFINE study. J Am Coll Cardiol 2007;50: 2275–2284.
- Zaman S, Narayan A, Thiagalingam A, Sivagangabalan G, Thomas S, Ross DL, etal. Long-term arrhythmia-free survival in patients with severe left ventricular dysfunction and no inducible ventricular tachycardia after myocardial infarction. Circulation 2014;129: 848–854.
- Daubert MA, White JA, Al – Khalidi HR, Velazquez EJ, Rao SV, Crowley AL, etal. Cardiac remodeling after large ST-elevation myocardial infarctionin the current therapeutic era. Am Heart J 2020;223: 87–97. 574. Chew DS, Heikki H, Schmidt G, Kavanagh KM, Dommasch M, Bloch Thomsen PE, etal. Change in left ventricular ejection fraction following first myocardial infarction and outcome. JACC Clin Electrophysiol 2018;4: 672–682.
- Bänsch D, Oyang F, Antz M, Arentz T, Weber R, Val – Mejias JE, etal. Successful catheter ablation of electrical storm after myocardial infarction. Circulation 2003;108: 3011–3016.
- Altmann DR, Mutschelknauss M, EhlN, Koller M, Schaer B, Jörg L, etal. Prevalence of severely impaired left ventricular ejection fraction after reperfused ST-elevation myocardial infarction. Swiss Med Wkly 2013;143: w13869.
- Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, etal. Declining risk of sudden death in heart failure. N Engl J Med 2017;377: 41–51.
- Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular prematurede polarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet 1997;349: 675–682.
- Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ, etal. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet 1997; 349: 667–674.
- Clemens M, Peich lP, Wichterle D, Pavlů L, Čihák R, Aldhoon B, etal. Catheter ablation of ventricular tachycardia as the first-line therapy in patients with coronary artery disease and preserved left ventricular systolic function: long-term results: VT ablation in patients with preserved LV function. J Cardiovasc Electrophysiol 2015;26: 1105–1110.
- Pacifico A, Hohnloser SH, Williams JH, Tao B, Saksena S, Henry PD, et al. Prevention of implantable – defibrillator shocks by treatment with sotalol. D, L-Sotalol Implantable Cardioverter-Defibrillator Study Group. N Engl J Med 1999; 340: 1855–1862.
- Willems S, Tilz RR, Steven D, Kääb S, Wegscheider K, GellérL, etal. Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantabledefibrillator (BERLINVT): a multicenter randomizedtrial. Circulation 2020;141: 1057–1067.
- Kuck K-H, Tilz RR, Deneke T, Hoffmann BA, Ventura R, Hansen PS, etal. Impact of substrate modification by catheter ablationon implantable cardioverter–defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: results from the multicenterr and omized controlled SMS (substratemodificationstudy). Circ Arrhythm Electrophysiol 2017;10: e004422. 584. Olshansky B, Hahn EA, Hartz VL, Prater SP, Mason JW. Clinical significance of syncope in the electrophysiologic study versus electrocardiographic monitoring (ESVEM) trial. The ESVEM Investigators. Am Heart J 1999; 137: 878–886.
- Molossi S, Agrawa lH, Mery CM, Krishnamurthy R, Masand P, Sexson Tejtel SK, etal. Outcomes in anomalous aortic origin of a coronary artery following aprospectivest and ardized approach. Circ Cardiovasc Interv 2020;13: e008445.
- Krasuski RA, Magyar D, Hart S, Kalahasti V, Lorber R, Hobbs R, etal. Long-term outcome and impact of surgery on in adults with coronary arteries originating from the opposite coronary cusp. Circulation 2011;123: 154–162
- Jegatheeswaran A, Devlin PJ, McCrindle BW, Williams WG, Jacobs ML, Blackstone EH, etal. Features associated with myocardial ischemia in anomalous aortic origin of a coronary artery: a congenital heart surgeons’society study. JThoracCardiovasc Surg2019;158: 822–834.
- Jegatheeswaran A, Devlin PJ, Williams WG, Brothers JA, Jacobs ML, De Campli WM, etal. Outcomes after anomalous aortic origin of acoronary artery repair: a congenital heart surgeons’ society study. J Thorac Cardiovasc Surg 2020;160: 757–771.
589.Hoffmayer KS, Bhave PD, Marcus GM, James CA, Tichnell C, Chopra N, etal. An electrocardiographic scoring system for distinguishing right ventricular outflow tract arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy from idiopathic ventricular tachycardia. Heart Rhythm2013; 10:477–482.
- Yokokawa M, Siontis KC, Kim HM, Stojanovska J, Latchamsetty R, Crawford T, etal. Value of cardiac magnetic resonance imaging and programmed ventricular stimulation in patients with frequent premature ventricular complexes undergoing radiofrequency ablation. Heart Rhythm 2017;14: 1695–1701.
- Muser D, Santangeli P, Castro SA, Casado Arroyo R, Maeda S, Benhayon DA, etal. Risk stratification of patients with apparently idiopathic premature ventricular contractions: a multicenter international CMR registry. JACCC lin Electrophysiol 2020;6: 722–735.
- Kjekshus J, Bathen J, Orning OM, Storstein L. Adouble-blind, cross over comparison of flecainide acetate and disopyramide phosphate in the treatment of ventricular premature complexes. Am J Cardiol 1984;53: 72B–78B.
- Hamon D, Swid MA, Rajendran PS, Liu A, Boyle NG, Shivkumar K, etal. Premature ventricular contraction diurnal profiles predict distinct clinical characteristics and beta-blocker responses. J Cardiovasc Electrophysiol 2019;30: 836–843.
- Primeau R, Agha A, Giorgi C, Shenasa M, Nadeau R. Longterm efficacy and toxicity of amiodarone in the treatment of refractory cardiac arrhythmias. Can J Cardiol 1989;5: 98–104. 595. Ling Z, Liu Z, Su L, Zipunnikov V, Wu J, Du H, etal. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythm Electrophysiol 2014;7: 237–243.
- Baksiene D, Sileikiene R, Sileikis V, Kazakevicius T, Zabiela V, Zebiene M, et al. Idiopathic ventricular tachycardia in children: curative therapy with radiofrequency ablation. Medicina (Kaunas) 2007;43: 803–807.
- Blaufox AD, Felix GL, Saul JP, Pediatric Catheter Ablation Registry. Radiofrequency catheter ablation in infants, /=18 month sold: when is it done and how do they fare ?: Short-termdatafrom the pediatric ablation registry. Circulation 2001; 104: 2803–2808.
- Lapage MJ, Bradley DJ, Dick M. Verapamil ininfants: an exaggerated fear? Pediatr Cardiol 2013;34: 1532–1534. 599. Lee AKY, Andrade J, Hawkins NM, Alexander G, Bennett MT, Chakrabarti S, etal. Outcomes of untreated frequent premature ventricular complexes with normal left ventricular function. Heart 2019; 105:1408–1413.
- Baman TS, Lange DC, Ilg KJ, GuptaSK, Liu T-Y, Alguire C, etal. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7: 865–869.
- vanHulsvanTaxis CFB, Piers SRD, de Riva Silva M, Dekkers OM, Pijnappels DA, Schalij MJ, etal. Fatigue as presenting symptom and a high burden of premature ventricular contractions are independently associated with increased ventricular rwall stress in patients with normal left ventricular function. Circ Arrhythm Electrophysiol 2015;8: 1452–1459.
- Sharma N, Cortez D, ImundoJ R. High burden of premature ventricular contractions in structurally normal hearts: to worry or not in pediatric patients? Ann Noninvasive Electrocardiol 2019;24: e12663.
- Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu M, etal. Prognostic significance of frequent premature ventricular contractions originating fromthe ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95: 1230–1237.
- Krittayaphong R, Sriratanasathavorn C, Dumavibhat C, Pumprueg S, Boonyapisit W, Poor an awattanakul S, etal. Electrocardiographic predictors of long-term outcome safter radiofrequency ablation in patients with right- ventricular outflow tract tachycardia. Europace 2006;8: 601–606.
- Komatsu Y, Nogami A, Kurosaki K, Morishima I, Masuda K, Ozawa T, et al. Fascicular ventricular tachycardia originating from papillary muscles: Purkinje network involvement in the reentrant circuit. Circ Arrhythm Electrophysiol 2017;10: e004549.
- Kirk CR, Gibbs JL, Thomas R, Radley- Smith R, Qureshi SA. Cardiovascular collapse after verapamil in supraventricular tachycardia. Arch Dis Child 1987;62: 1265–1266.
- Duffee DF, Shen WK, Smith HC. Suppression of frequent premature ventricular contractions and improvementof left ventricular function in patients with presummed idiopathic dilated cardiomyopathy. May Clin Proc 1998;73: 430–433.
- Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex – induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol2 000;11: 328–329.
- Penela D, Van Huls Van Taxis C, Van Huls Vans Taxis C, Aguinaga L, Fernández- Armenta J, Mon tL, etal. Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction: a prospective multicenter study. J am Coll Cardiol 2013;62: 1195–1202.
- Penela D, Acosta J, Aguinaga L, Tercedor L, Ordoñez A, Fernández-Armenta J, etal. Ablation of frequen tPVC in patients meeting criteria for primary preventionI CD implant: safety of with holding the implant. Heart Rhythm 2015;12: 2434–2442.
- Voskoboinik A, Hadjis A, Alhede C, Im SI, Park H, Moss J, etal. Predictors of adverse outcome in patients with frequent premature ventricular complexes: the ABC-VT riskscore. Heart Rhythm 2020;17: 1066–1074.
- LeeA, DenmanR, Haqqani HM. Ventricular ectopy in the context of left ventricular systolic dysfunction: risk factors and outcomes following catheter ablation. Heart Lung Circ 2019;28: 379–388.
- Sadron Blaye-Felice M, Hamon D, Sacher F, Pascale P, Rollin A, Duparc A, etal. Premature ventricular contraction-induced cardiomyopathy: related clinical and electrophysiologic parameters. Heart Rhythm 2016; 13:103–110.
- Penela D, Fernández-Armenta J, Aguinaga L, Tercedor L, Ordoñez A, Bisbal F, etal. Clinical recognition of pure premature ventricular complex-induced cardiomyopathy at presentation. Heart Rhythm 2017; 14:1864–1870.
- Aquaro GD, Pingitore A, Strata E, DiBella G, Molinaro S, Lombardi M. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J AmColl Cardiol 2010;56: 1235–1243.
- Oebel S, Dinov B, Arya A, Hilbert S, Sommer P, Bollmann A, etal. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J Cardiovasc Electrophysiol 2017;28: 1316–1323.
- Mountantonakis SE, Frankel DS, Gerstenfeld EP, Dixit S, Lin D, Hutchinson MD, etal. Reversal of outflow tract ventricular premature depolarization – induced cardiomyopathy with ablation: effectof residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm 2011;8: 1608–1614.
- Zang M, Zhang T, MaoJ, Zhou S, He B. Beneficial effects of catheter ablation of frequent premature ventricular complexeson left ventricular function. Heart2014; 100:787–793. 619. Wijnmaalen AP, Delgado V, Schalij MJ, van Hulsvan T axis CFB, Holman ER, Bax JJ, etal. Beneficial effects of catheter ablation on left ventricular and right ventricular function in patients with frequent premature ventricular contractions andpre served ejection fraction. Heart 2010; 96:1275–1280.
- Bogun F, CrawfordT, Reich S, Koelling TM, ArmstrongW, Good E, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm2007;4: 863–867.
- Sarrazin J-F, Labounty T, Kuhne M, Crawford T, Armstrong WF, Desjardins B, etal. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009;6: 1543–1549.
- ElKadri M, Yokokawa M, Labounty T, Mueller G, Crawford T, Good E, etal. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Heart Rhythm 2015;12: 706–713.
- Lakkireddy D, Di Biase L, Ryschon K, Biria M, Swarup V, Reddy YM, et al. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in non responders. J AmColl Cardiol 2012; 60:1531–1539.
- Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival trial of antiarrhythmic therapy in congestive heart failure. N Engl J Med1995; 333:77–82.
- Zhong L, Lee Y-H, Huang X-M, Asirvatham SJ, Shen W-K, Friedman PA, et al. Relative efficacy of catheter ablation vs antiarrhythmic drug sin treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 2014; 11: 187–193.
- Hyman MC, Mustin D, Supple G, Schaller RD, Santangeli P, Arkles J, etal. Class IC antiarrhythmic drugs for suspected premature ventricular contraction- induced cardiomyopathy. Heart Rhythm 2018;15: 159–163.
- Laurent G, Saal S, Amarouch MY, Béziau DM, Marsman RFJ, Faivre L, etal. Multifocal ectopic Purkinje- related premature contractions: a new SCN5A-related cardiac channelopathy. J Am Coll Cardiol 2012; 60: 144–156.
- Calloe K, Broendberg AK, Christensen AH, Pedersen LN, Olesen MS, deLos Angeles Tejada M, etal. Multifocal atrial and ventricular premature contractions with an increased risk of dilated cardiomyopathy caused by a Nav 1.5 gain-of-functionmutation (G213D). Int J Cardiol 2018;257: 160–167. 629. Mann SA, Castro ML, Ohanian M, Guo G, Zodgekar P, Sheu A, et al. R222Q SCN5A mutation is associated with reversible ventricular ectopy and dilated cardiomyopathy. J Am Coll Cardiol 2012;60: 1566–1573.
- Beckermann TM, McLeod K, Murday V, Potet F, George AL. Novel SCN5Amuta tioninamiodarone-responsive multifocal ventricular ectopy-associated cardiomyopathy. Heart Rhythm 2014;11: 1446–1453.
- Doisne N, Waldmann V, Redheuil A, Waintraub X, Fressart V, Ader F, etal. Anovel gain-of-function mutation in SCN5A responsible for multifocal ectopic Purkinje-related premature contractions. Hum Mutat 2020;41: 850–859.
- Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classificationof thecardiomyopathies: a position statement from the European Society Of Cardiology Working Groupon Myocardial and Pericardial Diseases. Eur Heart J2008;29: 270–276.
- Codd MB, Sugrue DD, Gersh BJ, Melton LJ. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. Apopulation-based study in Olmsted County, Minnesota, 1975–1984.Circulation1989;80:564–572.
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet2017; 390: 400–414.
- Beggs SAS, Jhund PS, Jackson CE, McMurray JJV, Gardner RS. Non-ischaemiccar diomyopathy, sudden death and implantable defibrillators: a review and meta-analysis. Heart 2018; 104:144–150.
- Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, etal. Incidence, causes, and outcomes of dilated cardiomyopathy inchildren. JAMA 2006; 296: 1867–1876.
- Pah lE, Sleeper LA, Canter CE, Hsu DT, Lu M, Webber SA, etal. Incidence of and risk factors for sudden cardiac death in children with dilated cardiomyopathy: are port from the Pediatric Cardiomyopathy Registry. J AmColl Cardiol 2012;59: 607–615.
- Bharucha T, Lee KJ, Daubeney PEF, Nugent AW, Turner C, Sholler GF, et al. Sudden death inchildhood cardiomyopathy: results from a long-term national population-based study. J am Coll Cardiol 2015;65: 2302–2310.
- Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, etal. Proposal for arevised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group onmyocardial and pericardial diseases. Eur Heart J 2016;37: 1850–1858.
- Asselbergs FW, Sammani A, Elliott P, Gimeno JR, Tavazzi L, Tendera M, et al. Differences between familial and sporadic dilated cardiomyopathy: ESCEORP Cardiomyopathy & Myocarditis registry. ESC Heart Fail 2021; 8:95–105.
- Ader F, DeGroote P, Réant P, Rooryck- Thambo C, Dupin – Deguine D, Rambaud C, etal. FLNC pathogenic variants in patients with cardiomyopathies: prevalence and genotype – phenotype correlations. Clin Genet 2019;96: 317–329.
- Kayvanpour E, Sedaghat-Hamedani F, Amr A, Lai A, Haas J, Holzer DB, et al. Genotype-phenotype associations indilated cardiomyopathy: meta-analysis on more than 8000 individuals. Clin Res Cardiol 2017; 106: 127–139.
- Ortiz – Genga MF, Cuenca S, DalFerro M, Zorio E, Salgado- Aranda R, Climent V, etal. Truncating FLNC mutations are associated with high- risk dilated and arrhythmogenic cardiomyopathies. J am Coll Cardiol 2016;68: 2440–2451.
- Vanden Hoogenh of MMG, Beqqali A, Amin AS, vander MadeI, Aufiero S, Khan MAF, etal. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calciumhandling. Circulation 2018;138: 1330–1342.
- Gigli M, Merlo M, Graw SL, Barbati G, Rowland TJ, Slavov DB, etal. Genetic risk of arrhythmic phenotypes in patients with dilated cardiomyopathy. J am Coll Cardiol 2019;74: 1480–1490.
- Heliö T, Elliott P, Koskenvuo JW, Gimeno JR, Tavazzi L, Tendera M, etal. ESCEORP cardiomyopathy registry: real-life practice of genetic counselling and testing in adult cardiomyopathy patients. ESC Heart Fail 2020; 7:3013–3021.
- Elming MB, Nielsen JC, Haarbo J, Videbæk L, Korup E, Signorovitch J, etal. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation 2017;136: 1772–1780.
- Kristensen SL, Levy WC, Shadman R, Nielsen JC, Haarbo J, Videbæk L, etal. Risk models for prediction of implantable cardioverter – defibrillator benefit. JACC Heart Fail 2019;7: 717–724.
- Wolff G, LinY, Karathanos A, Brockmeyer M, Wolters S, Nowak B, et al. Implantable cardioverter/defibrillators for primary prevention in dilated cardiomyopathy post-DANISH: an updated meta-analysis and systematic review of randomized controlled trials. Clin Res Cardiol 2017;106: 501–513.
- KadishA, Dyer A, Daubert JP, Quigg R, Estes NAM, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350: 2151–2158.
- Klem I, Klein M, Khan M, Yang EY, Nabi F, Ivanov A, etal. Relationship of LVEF and myocardial scar to long-term mortality risk and mode of death in patients with nonischemic cardiomyopathy. Circulation 2021; 143: 1343–1358.
- van RijsingenI AW, Arbustini E, Elliott PM, Mogensen J, Hermans-van AstJF, vander Kooi AJ, etal. Risk factors for malignant ventricularar rhythmias in lamina /cmutation carriers a European cohort study. J am Coll Cardiol 2012; 59:493–500.
- Thuillot M, Maupain C, Gandjbakhch E, Waintraub X, Hidden – Lucet F, Isnard R, etal. External validation of risk factors for malignant ventricular arrhythmias in lamin A/Cmutationcarriers.Eur JHeartFail2019;21:253–254.
- Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, etal. Long-term outcome and risk stratification indilated cardiolaminopathies. J AmColl Cardiol 2008; 52: 1250–1260.
- Skjølsvik ET, Hasselberg NE, Dejgaard LA, Lie ØH, Andersen K, Holm T, etal. Exercise isassociated with impaired left ventricular systolic function inpatients withlaminA/Cgenotype. J am Heart Assoc2020;9: e012937.
- Verstraelen TE, vanLint FHM, Bosman LP, de Brouwer R, Proost VM, Abeln BGS, etal. Prediction of ventricular arrhythmia in phospholambanp. Arg 14 delmutation carriers-reaching the frontiers of individual riskprediction. EurHeart J2021;42: 2842–2850.
- Knight BP, Goyal R, Pelosi F, Flemming M, Horwood L, Morady F, etal. Outcome of patients with nonischemic dilated cardiomyopathy and unexplained syncope treated with an implantable defibrillator. J Am Coll Cardiol 1999;33: 1964–1970.
- Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA 2004; 292:2874–2879.
- Merlo M, Gentile P, Artico J, Cannatà A, Paldino A, De Angelis G, etal. Arrhythmic risk stratification in patients with dilated cardiomyopathy and intermediate left ventricular dysfunction. J Cardiovasc Med (Hagerstown) 2019;20: 343–350.
- Zecchin M, DiLenarda A, Gregori D, Merlo M, Pivetta A, Vitrella G, etal. Arenon sustained ventricular tachycardias predictive of major arrhythmias in patients with dilated cardiomyopathy on optimal medical treatment? Pacing Clin Electro 2008; 31: 290–299.
- Link MS, Costeas XF, Griffith JL, Colburn CD, Estes NA, Wang PJ. High incidence of appropriate implantable cardioverter – defibrillator therapy in patients with syncope of unknown etiology and inducible ventricular arrhythmias. J Am Coll Cardiol 1997; 29: 370–375.
- Di Marco A, Brown PF, Bradley J, Nucifora G, Claver E, de Frutos F, etal. Improved risk stratification for ventricular arrhythmias and sudden death in patients withnon ischemicdilatedcardiomyopathy. J Am Coll Cardiol 2021; 77: 2890–2905.
- Kumar S, Romero J, Mehta NK, Fujii A, Kapur S, Baldinger SH, etal. Long-termout comes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm 2016;13: 1957–1963.
- Muser D, Santangeli P, Castro SA, Pathak RK, Liang JJ, Hayashi T, etal. Long-term outcome after catheter ablation of ventricular tachycardia in patients with nonischemic dilated cardiomyopathy. Circ Arrhythm Electrophysiol 2016;9: e004328.
- Kumar S, Androulakis AFA, Sellal J-M, Maury P, Gandjbakhch E, Waintraub X, etal. Multicenter experience with catheter ablation for ventricular tachycardia in lamin A/C cardiomyopathy. Circ Arrhythm Electrophysiol 2016;9: e004357.
- Oloriz T, Silberbauer J, Maccabelli G, Mizuno H, Baratto F, Kirubakaran S, etal. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus inferolateral scar sub-types. CircArrhythmElectrophysiol 2014;7: 414–423.
- Francone M. Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution and prognostic significance. ISRNRadiol2014; 2014:365404.
- Goldberger JJ, Subačius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J am Coll Cardiol 2014;63: 1879–1889.
- Ebert M, Wijnmaalen AP, deRiva M, Trines SA, And roulakis AFA, Glashan CA, etal. Prevalence and prognostic impact of pathogenic variants in patients with dilated cardiomyopathy referred for ventricular tachycardia ablation. JACC Clin Electrophysiol2020;6: 1103–1114.
- Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017; 376:1489–1490. 671. Quarta G, Muir A, Pantazis A, Syrris P, Gehmlich K, Garcia-PaviaP, etal. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: impact of genetics andrevised task force criteria. Circulation2011;123: 2701–2709.
- Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichne llC, Jongbloed JDH, etal. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/ cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36: 847–855.
- RigatoI, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, etal. Compound and digenicheterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomalgene – related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 2013; 6: 533–542.
- te Riele ASJM, James CA, Groeneweg JA, Sawant AC, Kammers K, Murray B, etal. Approach to family screening in arrhythmogenic right ventricular dysplasia /cardio myopathy. Eur Heart J2016;37: 755–763
- Chivulescu M, Lie ØH, Popescu BA, Skulstad H, Edvardsen T, Jurcut RO, etal. High penetrance and similar disease progression in probands and infamily members with arrhythmogenic cardiomyopathy. Eur Heart J 2020;41: 1401–1410.
- Rastegar N, TeRiele ASJM, James CA, Bhonsale A, Murray B, Tichne llC, etal. Fibrofatty changes: incidence at cardiac MR imaging in patients with arrhythmogenic right ventricular dysplasia / cardiomyopathy. Radiology 2016; 280:405–412.
- Aquaro GD, Barison A, Todiere G, Grigoratos C, AitAli L, Di Bella G, et al. Usefulness of combined functional assessment by cardiacmagnetic resonance and tissue characterization versus task force criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2016; 118:1730–1736.
- teRiele ASJM, Bhonsale A, James CA, Rastegar N, Murray B, Burt JR, et al. Incremental value of cardiac magnetic resonance imaging in arrhythmic risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy- associated desmosomal mutation carriers. J am Coll Cardiol 2013;62: 1761–1769.
- Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, etal. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J 2020;41: 1414–1429.
- Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia /cardiomyopathy provides novel in sights into patterns of disease expression. Circulation 2007; 115:1710–1720.
681.Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin S, et al. Arrhythmogenic right ventricular cardiomyopathy: characterizationof left ven tricularphenotypeanddifferential diagnosiswithdilatedcardiomyopathy. J Am HeartAssoc2020;9: e014628.
- Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari MD, etal. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020; 319: 106–114.
- Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, SmithH, etal. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16: 1337–1344.
- LieØ H, Dejgaard LA, Saberniak J, Rootwelt C, Stokke MK, Edvardsen T, et al. Harmful effects of exercise intensity and exercise duration in patients with arrhythmogenic cardiomyopathy. JACC Clin Electrophysiol 2018;4: 744–753.
- James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, etal. Exercise increasesage-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62: 1290–1297. 686. Ruwald A-C, Marcus F, Estes NAM, Link M, McNitt S, Polonsky B, etal. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J2015;36: 1735–1743.
- Sawant AC, te Riele ASJM, Tichnell C, Murray B, Bhonsale A, Tandri H, etal. Safety ofAmericanHeartAssociation-recommendedminimumexercisefordesmosomal mutationcarriers. Heart Rhythm 2016; 13:199–207. 688. ThieneG, Nava A, Corrado D, RossiL, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Eng l J Med 1988; 318:129–133.
- Finocchiaro G, Papadakis M, Robertus J-L, Dhutia H, Steriotis AK, Tome M, etal. Etiology of sudden death in sports: insights fromaUnitedKingdomRegional Registry. J am Coll Cardiol 2016; 67: 2108–2115.
- Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, etal. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol 2009; 54:609–615.
691.HulotJ-S, Jouven X, Empana J-P, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 2004; 110:1879–1884.
- Wang W, Cadrin-Tourigny J, Bhonsale A, Tichnell C, Murray B, Monfredi O, etal. Arrhythmic outcome of arrhythmogenic right ventricular cardiomyopathy patients without implantable defibrillators. J Cardiovasc Electrophysiol 2018; 29:1396–1402.
- Pinamonti B, Dragos AM, Pyxaras SA, Merlo M, Pivetta A, Barbati G, et al. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10-year registry. Eur Heart J 2011;32: 1105–1113.
- Brun F, Groeneweg JA, Gear K, Sinagra G, vander Heijden J, Mestroni L, etal. Risk stratification in arrhythmic right ventricular cardiomyopathy without implantable cardioverter-defibrillators. JACC Clin Electrophysiol 2016;2: 558–564.
- Bhonsale A, James CA, Tichnell C, Murray B, Gagarin D, Philips B, etal. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation forprimaryprevention. J AmColl Cardiol 2011;58: 1485–1496.
- Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, etal. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillationor sustained ventricular tachycardia. Circulation 2010;122: 1144–1152.
- Platonov PG, Haugaa KH, Bundgaard H, Svensson A, Gilljam T, Hansen J, etal. Primary prevention of sudden cardiac deathwith implantable cardioverter defibrillat or therapy in patients with arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2019; 123:1156–1162.
- LinkMS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, etal. Ventricularar rhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol 2014; 64:119–125.
- Schinkel AFL. Implantable cardioverter defibrillators in arrhythmogenic right ventricular dysplasia/cardiomyopathy: patient outcomes, incidence of appropriate and in appropriate interventions, and complications. Circ Arrhythm Electrophysiol 2013;6: 562–568.
- Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, etal. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2003; 108: 3084–3091.
- Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N, et al. Arrhythmogenic right ventricular cardiomyopathy: clinical course and predictors of arrhythmic risk. J am CollCardiol 2016;68: 2540–2550.
- Cadrin-Tourigny J, Bosman LP, Wang W, Tadros R, Bhonsale A, Bourfiss M, etal. Sudden cardiac death prediction in arrhythmogenic right ventricular cardiomyopathy: a multinational collaboration. Circ Arrhythm Electrophysiol 2021;14: e008509.
- Bosman LP, Sammani A, James CA, Cadrin-Tourigny J, Calkins H, van Tintelen JP, etal. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. Heart Rhythm 2018;15: 1097–1107.
- Saguner AM, Vecchiati A, Baldinger SH, Rüeger S, Medeiros-Domingo A, Mueller-Burri AS, etal. Different prognostic value of functional rightventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Cardiovasc Imaging 2014;7: 230–239.
- Saguner AM, Medeiros-Domingo A, Schwyzer MA, On C-J, Haegeli LM, Wolber T, etal. Usefulness of inducible ventricular tachycardia to predict long-term adverse outcomes in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2013;111: 250–257.
- Orgeron GM, Te Riele A, Tichnell C, Wang W, Murray B, Bhonsale A, et al. Performanceofthe2015international task force consensus statement risk stratification algorithm for implantable cardioverter-defibrillator placement inarrhyth mogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol 2018;11: e005593.
- Orgeron GM, James CA, Te Riele A, Tichnell C, Murray B, BhonsaleA, et al. Implantable cardioverter-defibrillator therapy in arrhythmogenic right ventricular dysplasia/cardiomyopathy: predictors of appropriate therapy, outcomes, and complications. J Am Heart Assoc 2017;6: e006242.
- Wichter T, Borggrefe M, Haverkamp W, Chen X, Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation 1992; 86:29–37.
- Mahida S, Venlet J, Saguner AM, Kumar S, Baldinger SH, Abdel Wahab A, et al. Ablation compared with drug therapy for recurrent ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy: results from a multicenter study. Heart Rhythm 2019; 16: 536–543.
- Ermakov S, Gerstenfeld EP, Svetlichnaya Y, Scheinman MM. Use of flecainide in combination antiarrhythmic therapy in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2017;14: 564–569.
- Canpolat U, Kabakçi G, Aytemir K, Dural M, Şahiner L, Yorgun H, etal. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia: frQRS and outcomes in ARVC/D. J Cardiovasc Electrophysiol 2013; 24:1260–1266.
- Martin A, Crawford J, Skinner JR, Smith W. High arrhythmic burden but low mortality during long-term follow-up in arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ 2016;25: 275–281.
- Santangeli P, Dello Russo A, Pieroni M, Casella M, DiBiase L, Burkhardt JD, etal. Fragmented and delayed electrograms within fibrofatty scar predict arrhythmic events in arrhythmogenic right ventricular cardiomyopathy: Results fromapro spective risk stratification study. Heart Rhythm 2012; 9:1200–1206.
- Berruezo A, Fernández-Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, etal. Combined endocardial and epicardial catheter ablation inarrhythmogenicright ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol 2012;5: 111–121.
- Ommen SR, Mita lS, Burke MA, Day SM, Deswa lA, Elliott P, etal. 2020AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a reportof theAmericanCollegeofCardiology/ AmericanHeartAssociation Joint Committee on Clinical Practice Guidelines. Circulation 2020; 142: e533–e557.
- Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, etal. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130: 484–495.
- He D, Ye M, Zhang L, Jiang B. Prognosticsignificanceof late gadolinium enhancement on cardiac magnetic resonance in patients with hypertrophic cardiomyopathy. Heart Lung 2018;47: 122–126.
- Weissler-Snir A, Dorian P, Rakowski H, Care M, Spears D. Primary prevention implantable cardioverter – defibrillators in hypertrophic cardiomyopathy— are there predictors of appropriate therapy? Heart Rhythm 2021;18: 63–70.
- Khanna S, Wen I, Bhat A, Chen HHL, Gan GCH, Pathan F, etal. The role of multi modality imaging in the diagnosis of cardiac amyloidosis: a focused update. Front Cardiovasc Med2020; 7:590557.
- Pradella S, Grazzini G, De Amicis C, Letteriello M, Acquafresca M, Miele V. Cardiac magnetic resonance in hypertrophic and dilated cardiomyopathies. Radiol Med 2020;125: 1056–1071.
- Rosmini S, Biagini E, O’Mahony C, Bulluck H, Ruozi N, Lopes LR, etal. Relationship between aetiology and left ventricular systolic dysfunction in hypertrophic cardiomyopathy. Heart2017;103: 300–306. 722. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, etal. Genotypeand lifetimeburdenof disease inhypertrophic cardiomyopathy: insights fromthe Sarcomeric HumanCardiomyopathy Registry (SHaRe). Circulation 2018;138: 1387–1398.
- KimHY, Park JE, Lee S-C, Jeon E-S, On YK, Kim SM, etal. Genotype-related clinical characteristics and myocardial fibrosis and their association with prognosis in hypertrophic cardiomyopathy. J Clin Med 2020;9: 1671.
- Maron BJ, Maron MS, Semsarian C. Double or compound sarcomere mutations in hypertrophic cardiomyopathy: a potential link to sudden death in the absence of conventional risk factors. Heart Rhythm 2012; 9:57–63.
- Wang J, Wang Y, Zou Y, Sun K, Wang Z, Ding H, etal. Malignant effects of multiple rarevariants in sarcomere genes on the prognosis of patients with hypertrophic cardiomyopathy. Eur J Heart Fail 2014; 16:950–957.
- Semsarian C, InglesJ, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65: 1249–1254.
- Maron BJ, Rowin EJ, Casey SA, Link MS, Lesser JR, Chan RHM, etal. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J am Coll Cardiol 2015; 65:1915–1928.
- O’Mahony C, Jichi F, Ommen SR, Christiaans I, ArbustiniE, Garcia-PaviaP, etal. International External Validation Study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention inHypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation 2018;137: 1015–1023.
- Vriesendorp PA, Schinkel AFL, Liebregts M, Theuns DAMJ, van Cleemput J, ten Cate FJ, etal. Validationof the 2014 European Society of Cardiology guidelines risk prediction model for the primary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol 2015;8: 829–835.
- LorenziniM, Anastasiou Z, O’Mahony C, Guttman OP, Gimeno JR, Monserrat L, etal. Mortality among referral patients with hypertrophic cardiomyopathy vs the general European population. JAMA Cardiol 2020;5: 73–80.
731.Maron BJ, Rowin EJ, Casey SA, Haas TS, Chan RHM, Udelson JE, etal. Risk stratification and outcome of patients with hypertrophic cardiomyopathy. = 60 years of age. Circulation 2013; 127:585–593.
- Gimeno JR, Tomé-Esteban M, Lofiego C, Hurtado J, Pantazis A, Mist B, et al. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur Heart J2009; 30:2599–2605.
- Pelliccia A, Lemme E, Maestrini V, Di Paolo FM, Pisicchio C, Di Gioia G, etal. Does sport participation worsen the clinical course of hypertrophic cardiomyopathy? Clinical outcome of hypertrophic cardiomyopathy in athletes. Circulation 2018; 137: 531–533. 734. Dejgaard LA, Haland TF, Lie OH, Ribe M, Bjune T, Leren IS, etal. Vigorous exercise in patients with hypertrophic cardiomyopathy. Int J Cardiol 2018; 250:157–163.
- Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKennaWJ. Non – sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003; 42:873–879. 736. Rowin EJ, Maron BJ, Carrick RT, Patel PP, Koethe B, Wells S, etal. Outcomes in patients with hypertrophic cardiomyopathy and left ventricular systolic dysfunc tion. J Am Coll Cardiol 2020;75: 3033–3043.
- Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, etal. Hypertrophic cardiomyopathy with left ventricular apicalaneurysm: implications for risk stratification and management. J Am Coll Cardiol 2017;69: 761–773. 738. Sadoul N, PrasadK, Elliott PM, Bannerjee S, FrenneauxMP, McKenna WJ. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation 1997; 96: 2987–2991.
- Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, etal. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 2000; 36:2212–2218. 740. Behr ER, Elliott P, McKenna WJ. Role of invasive EP testing in the evaluation and management of hypertrophic cardiomyopathy. Card Electrophysiol Rev 2002;6: 482–486.
- Gatzoulis KA, Georgopoulos S, Antoniou C-K, Anastasakis A, Dilaveris P, Arsenos P, etal. Programmed ventricular stimulation predicts arrhythmic events and survival in hypertrophic cardiomyopathy. Int J Cardiol 2018; 254:175–181.
- Norrish G, Qu C, Field E, Cervi E, Khraiche D, Klaassen S, etal. External validation of the HCM Risk-Kids model for predicting sudden cardiac death in childhood hypertrophic cardiomyopathy. Eur J Prev Cardiol 2022;29: 678–686.
- Petryka – Mazurkiewicz J, Ziolkowska L, Kowalczyk-Domagala M, MazurkiewiczL, Boruc A, Spiewak M, etal. LGE for risk stratification in primary prevention in children with HCM. JACC Cardiovasc Imaging 2020;13: 2684–2686.
- Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J am Coll Cardiol 1999;33: 1596–1601.
- Cecchi F, Maron BJ, Epstein SE. Long-term outcome of patients with hypertrophic cardiomyopathy successfully resuscitatedafter cardiac arrest. J AmColl Cardiol 1989;13: 1283–1288.
- Maron BJ, Spirito P, ShenW-K, Haas TS, Formisano F, Link MS, etal. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007;298: 405–412.
- Melacini P, Maron BJ, Bobbo F, Basso C, Tokaju kB, Zucchetto M, etal. Evidence that pharmacological strategies lack efficacy for the prevention of sudden death in hypertrophic cardiomyopathy. Heart 2007;93: 708–710.
- McKenna WJ, Oakley CM, Krikler DM, Goodwin JF. Improved survival with amio darone in patients with hypertrophic cardiomyopathy and ventricular tachycardia. BrHeart J 1985; 53:412–416.
- Link MS, Bockstall K, Weinstock J, Alsheikh-AliAA, Semsarian C, Estes NAM, etal. Ventricular tachyarrhythmias inpatientswithhypertrophiccardiomyopathyand defibrillators: triggers, treatment, and implications. J Cardiovasc Electrophysiol 2017; 28:531–537.
750.Dallaglio PD, diMarco A, Moreno Weidmann Z, Perez L, Alzueta J, García-Alberola A, etal. Antitachycardia pacing for shock prevention in patients with hypertrophic cardiomyopathy and ventricular tachycardia. Heart Rhythm 2020; 17:1084–1091.
- AdduciC, Semprini L, Palano F, Musumeci MB, Volpe M, Autore C, etal. Safety and efficacy of anti-tachycardia pacing in patients with hypertrophic cardiomyopathy implanted with an ICD. Pacing Clin Electrophysiol 2019;42 :610–616.
- Vaseghi M, Hu TY, Tung R, Vergara P, Frankel DS, Di BiaseL, etal. Outcomes of catheter ablation of ventricular tachycardia based on etiology in non ischemic heart disease: an international ventricular tachycardia ablation center collaborative study. JACC Clin Electrophysiol 2018;4: 1141–1150.
- Igarashi M, Nogami A, Kurosaki K, Hanaki Y, KomatsuY, Fukamizu S, et al. Radio frequency catheter ablation of ventricular tachycardia in patients with hypertrophic cardiomyopathy and apical aneurysm. JACCClin Electrophysiol 2018;4: 339–350.
- Dukkipati SR, d’Avila A, Soejima K, Bala R, Inada K, Singh S, etal. Long-term outcomes of combined epicardial and endocardial ablation of monomorphic ventricular tachycardia related to hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4: 185–194.
- Ross SB, Singer ES, Driscoll E, Nowak N, Yeates L, Puranik R, etal. Genetic architecture of left ventricular noncompaction in adults. Hum Genome Var 2020;7: 33.
- Weir- Mc Call JR, Yeap PM, Papagiorcopulo C, Fitzgerald K, Gandy SJ, Lambert M, etal. Left ventricular non compaction: anatomical phenotype or distinct cardiomyopathy? J Am Coll Cardiol 2016;68: 2157–2165.
- Aung N, Doimo S, Ricci F, Sanghvi MM, Pedrosa C, Wood bridge SP, et al. Prognostic significance of left ventricular non compaction: systematic review and meta- analysis of observational studies. Circ Cardiovasc Imaging 2020; 13: e009712.
- Grigoratos C, Barison A, Ivanov A, Andreini D, Amzulescu M-S, MazurkiewiczL, etal. Meta-analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular non compaction. JACC Cardiovasc Imaging 2019;12: 2141–2151.
- Richard P, Ader F, Roux M, Donal E, Eicher J-C, Aouti lN, etal. Targeted panelse quenching in adult patients with left ventricular non-compaction reveal salargegen eticheterogeneity. Clin Genet 2019; 95:356–367.
- Seferović PM, Polovina M, Bauersachs J, Arad M, Gal TB, Lund LH, etal. Heartfailure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21:553–576. 761. BaigS, Edward NC, Kotecha D, LiuB, Nordin S, Kozor R, etal. Ventricular arrhythmia and sudden cardiac death in Fabry disease: a systematic review of risk factors in clinical practice. Europace 2018;20: f153–f161.
- Sperry BW, Ikram A, Hachamovitch R, Valent J, Vranian MN, Phelan D, etal. Efficacy of chemotherapy for light-chain amyloidosis in patients presenting with symptomatic heart failure. J am Coll Cardiol 2016;67: 2941–2948.
- Kristen AV, Dengler TJ, Hegenbart U, Schonland SO, Goldschmidt H, Sack F- U, etal. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 2008;5: 235–240.
- Merino JL, Peinado R. Arrhythmias associated with neuromuscular disorders. Card Electrophysiol Rev 2002;6: 132–135.
- Punnoose AR, Kaltman JR, Pastor W, Mc Carter R, He J, Spurney CF. Cardiacdis ease burden and risk of mortality in hospitalized muscular dystrophy patients. Pediatr Cardiol 2016;37: 1290–1296. 766.Wahbi K, Meune C, Porcher R, Bécane HM, Lazarus A, Laforêt P, et al. Electrophysiologic a study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA 2012; 307:1292–1301.
- Lallemand B, Clementy N, Bernard-Brunet A, Pierre B, Corcia P, FauchierL, etal. The evolution of infrahissian conduction time in myotonic dystrophy patients: clinical implications. Heart2012; 98:291–296.
- Nogami A. Bundle branch reentry tachycardia. ESC CardioMed. 3rded. Oxford University Press; 2022, pp.2270–2275.
- Sanna T, DelloRusso A, Toniolo D, Vytopil M, Pelargonio G, De Martino G, etal. Cardiac features of Emery- Dreifuss muscular dystrophy caused by lamin A / C gene mutations. EurHeart J 2003; 24:2227–2236.
- Menon SC, Etheridge SP, Liesemer KN, Williams RV, Bardsley T, Heywood MC, etal. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. PediatrCardiol 2014; 35:1279–1285.
- Florian A, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U, etal. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson2014; 16:81.
- Prystowsky EN, Pritchett EL, Roses AD, Gallagher J. The natural history of conduction system disease in myotonic muscular dystrophy as determined by serial electrophysiologic studies. Circulation 1979;60: 1360–1364.
- Trachtenberg BH, Hare JM. Inflammatory cardiomyopathic syndromes. CircRes 2017;121: 803–818.
- Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, etal. Macrophages facilitate electrical conduction in the heart. Cell 2017;169: 510–522.
- Youker KA, Assad-Kottner C, Cordero-Reyes AM, Trevino AR, Flores – Arredondo JH, Barrios R, etal. High proportion of patients with end-stage heart failure regard less of aetiology demonstrates anti-cardiac antibody deposition in failing myocardium: humoral activation, a potential contributor of disease progression. Eur Heart J2014; 35:1061–1068.
- Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflam mationin heart failure. Nat Rev Cardiol 2020; 17:269–285.
- Sagar S, Liu PP, Cooper LT. Myocarditis. Lancet 2012;379: 738–747.
- Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, etal. Clinical presentation and outcome in acontemporary cohort of patients with acute myocarditis: multicenter Lombardy registry. Circulation 2018;138: 1088–1099.
- Fabre A. Sudden adult death syndrome and other non – ischaemic causes of sudden cardiac death. Heart 2005;92: 316–320.
- Lynge TH, Nielsen TS, Gregers Winkel B, T felt- Hansen J, Banner J. Sudden cardiac death caused by myocarditis in persons aged 1– 49 years: anation wide study of 14 294 deaths in Denmark. Forensic Sci Res 2019;4: 247–256.
- Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NAM, etal. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis. J Am Coll Cardiol 2015; 66: 2362–2371.
- Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, etal. Update on myocarditis. J am Coll Cardiol 2012;59: 779–792. 783.CaforioALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, etal. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. EurHeart J 2013;34: 2636–2648.
- Singh V, Mendirichaga R, Savani GT, Rodriguez A, Blumer V, Elmariah S, et al. Comparison of utilization trends, indications, and complications ofendomyocardial biopsy in native versus do nor hearts (from the Nation wide In patient Sample 2002 to 2014). Am J Cardiol 2018; 121:356–363.
- Aoyama N, Izumi T, Hiramori K, Isobe M, Kawana M, Hiroe M, etal. National survey of fulminant myocarditis in Japan: therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (specialre port from a scientific committee). Circ J 2002; 66:133–144.
- Shah Z, Mohammed M, Vuddanda V, Ansari MW, Masoomi R, Gupta K. National trends, gender, management, and outcomes of patients hospitalized for myocarditis. Am J Cardiol 2019;124: 131–136.
- Anderson BR, Silver ES, Richmond ME, Liberman L. Usefulness of arrhythmias as predictors of death and resource utilization in children with myocarditis. Am J Cardiol 2014;114: 1400–1405.
- Kandolin R, Lehtonen J, Salmenkivi K, Räisänen-Sokolowski A, Lommi J, Kupari M. Diagnosis, treatment, and outcome of giant-cellmyocarditis in the era of combined immunosuppression. Circ Heart Fail 2013;6: 15–22.
- Cooper LT, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis— natural history and treatment. N Engl J Med 1997;336: 1860–1866.
- McMurrayJ JV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, etal. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33: 1787–1847. 791. Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis 2010; 52:274–288.
- JCS Joint Working Group. Guidelines for diagnosis and treatment of myocarditis (JCS2009): digestversion. Circ J 2011; 75:734–743.
- Ali- Ahmed F, Dalgaard F, Al-Khatib SM. Sudden cardiac death in patients with myocarditis: Evaluation, risk stratification, andmanagement. Am Heart J 2020;220: 29–40.
- Rosier L, Zouaghi A, Barré V, Martins R, Probst V, Marijon E, etal. High risk of sus tained ventricular arrhythmia recurrence after acute myocarditis. J Clin Med 2020;9: E848.
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Councilon Epidemiology and Prevention. Circulation 2006; 113: 1807–1816.
- D’Ambrosio A, Patti G, Manzoli A, Sinagra G, DiLenarda A, Silvestri F, etal. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 2001;85: 499–504.
- Kasper EK, Agema WRP, Hutchins GM, Deckers JW, Hare JM, Baughman KL. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J Am Coll Cardiol 1994; 23:586–590.
- Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, etal. Long-term follow-up of biopsy– proven viral myocarditis. J Am Coll Cardiol 2012; 59: 1604–1615.
- Bennett MK, Gilotra NA, Harrington C, Rao S, Dunn JM, Freitag TB, etal. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from2000–2009. Circ Heart Fail 2013;6: 676–684.
- Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, etal. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson 2014;16: 14.
- Russo AD, Casella M, Pieroni M, Pelargonio G, Bartoletti S, Santangeli P, et al. Drug-refractory ventricular tachycardias after myocarditis: endocardial and epicardial radiofrequency catheter ablation. Circ Arrhythm Electrophysiol 2012; 5:492–498.
- Maccabelli G, Tsiachris D, Silberbauer J, Esposito A, Bisceglia C, Baratto F, etal. Imaging andepicardial substrate ablationof ventricular tachycardia inpatients late after myocarditis. Europace 2014;16: 1363–1372.
- Berte B, Sacher F, Cochet H, Mahida S, Yamashita S, Lim H, etal. Post myocarditis ventricular tachycardia in patients with epicardial – only scar: aspecificentity requiring a specific approach: epicardial-only VT ablation. J Cardiovasc Electrophysiol 2015; 26:42–50.
- Maleszewski JJ, Orellana VM, Hodge DO, Kuh lU, SchultheissH-P, Cooper LT. Long-termrisk of recurrence, morbidity and mortality ingiant cellmyocarditis. Am J Cardiol 2015;115: 1733–1738.
- El-AssaadI, Al-Kindi SG, Oliveira GH, Boyle GJ, Aziz PF. Implantable cardioverter defibrillator and wait-list outcomes in pediatric patients awaiting heart transplant ation. Heart Rhythm 2015;12: 2443–2448.
- Ekström K, Lehtonen J, Kandolin R, Räisänen-Sokolowski A, Salmenkivi K, Kupari M. Long-term outcome and its predictors in giant cell myocarditis: giant cell myocarditis. Eur JHeart Fail 2016;18: 1452–1458.
- Rybicki BA, Iannuzzi MC, Frederick MM, Thompson BW, Rossman MD, Bresnitz EA, etal. Familial aggregation of sarcoidosis. A case – control etiologic study of sarcoidosis (ACCESS). Am JR espir Crit Care Med 2001; 164: 2085–2091.
- Muchtar E, Blauwet LA, Gertz MA. Restrictive cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 2017;121: 819–837.
- Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, etal. HRS expert consensus statement on the diagnosis and management of arrhythmias as sociated with cardiac sarcoidosis. Heart Rhythm 2014;11: 1304–1323.
- Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J 2016;38: 2663–2670.
- Trivieri MG, Spagnolo P, Birnie D, Liu P, Drake W, Kovacic JC, etal. Challengesin cardiac and pulmonary sarcoidosis: JACC state – of – the – art review. J Am Coll Cardiol 2020;76: 1878–1901.
- Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, etal. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years inanation wide study. Circulation 2015;131: 624–632.
- Hoogendoorn JC, SramkoM, Venlet J, Siontis KC, Kumar S, SinghR, et al. Electroanatomical voltage mapping to distinguish right – sided cardiac sarcoidosis from arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol 2020; 6:696–707.
- Banba K, KusanoKF, Nakamura K, MoritaH, Ogawa A, Ohtsuka F, et al. Relationshipbetween arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm 2007;4: 1292–1299. 815. Takaya Y, Kusano KF, Nakamura K, Ito H. Outcomes in patients with high – degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. Am J Cardiol 2015; 115:505–509.
- Nordenswan H-K, Lehtonen J, Ekström K, Kandolin R, Simonen P, Mäyränpää M, etal. Outcome of cardiac sarcoidosis presenting with high – grade atrioventricular block. Circ Arrhythm Electrophysiol 2018;11.
- Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, etal. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013;6: 501–511.
- Nade lJ, Lancefield T, Voskoboinik A, Taylor AJ. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-termventricular arrhythmia and sudden cardiac death. EurHeart J Cardiovasc Imaging 2015; 16:631–641.
- Murtagh G, LaffinL J, Beshai JF, Maffessanti F, Bonham CA, Patel AV, etal. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascularmagnetic resonance. Circ Cardiovasc Imaging 2016;9: e003738. 820. Ekström K, Lehtonen J, Hänninen H, Kandolin R, Kivistö S, Kupari M. Magnetic resonance imaging as a predictor of survival free of life – threatening arrhythmias and transplantation in cardiac sarcoidosis. J Am Heart Assoc 2016;5: e003040.
821.ColemanGC, Shaw PW, Balfour PC, Gonzalez JA, Kramer CM, Patel AR, etal. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging 2017; 10:411–420.
- AizerA, Stern EH, Gomes JA, Teirstein AS, Eckart RE, Mehta D. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. Am J Cardiol 2005; 96:276–282.
- Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: roleofprogrammed ventricular stimulation. Circ Arrhythm Electrophysio l2011; 4:43–48.
824.OkadaDR, Smith J, Derakhshan A, Gowani Z, Zimmerman SL, Misra S, et al. Electrophysiology study for risk stratification in patients with cardiac sarcoidosis and abnormal cardiac imaging. Int J CardiolHeartVasc 2019;23: 100342.
- Zipse MM, Tzou WS, Schuller JL, AleongRG, Varosy PD, Tompkins C, et al. Electrophysiologic testing for diagnostic evaluation and risk stratification in patients with suspected cardiac sarcoidosis with preserved left and right ventricular systolic function. J Cardiovasc Electrophysiol 2019;30: 1939–1948.
- Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, etal. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014; 63:329–336.
- Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, GuX, et al. Magnetic resonance imaging for identifyingpatientswithcardiacsarcoidosisand preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014; 7:1109–1115.
- Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, etal. Implantablecar dioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol 2012; 23:925–929.
- Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, et al. Long – termfollow –up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm 2012; 9:884–891.
- Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, etal. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. EP Europace 2013;15: 347–354.
- Mohsen A, Jimenez A, Hood RE, Dickfeld T, Saliaris A, Shorofsky S, etal. Cardiac sarcoidosis: electrophysiological outcomeson long-termfollow-upandtherole of the implantablecardioverter-defibrillator. J CardiovascElectrophysiol 2014;25: 171–176.
- AzoulayL-D, Waintraub X, Haroche J, Amoura Z, Cohen Aubart F. Factor sassociated with implantable cardioverter defibrillators appropriate therapy incardiac sarcoidosis: ameta-analysis: implantable cardioverter defibrillators in cardiacsar coidosis. Sarcoidosis Vasc Diffuse Lung Dis 2020; 37:17–23.
- SmedemaJ -P, vanGeuns R-J, Ector J, Heidbuche lH, Ainslie G, CrijnsHJ GM. Right ventricular involvement and the extent of left ventricular enhancement with magnetic resonance predict adverse outcome in pulmonary sarcoidosis. ESC Heart Fail 2018; 5:157–171.
- Velangi PS, ChenK-HA, Kazmircza kF, Okasha O, von Wald L, Roukoz H, etal. Right ventricular abnormalities on cardiovascular magnetic resonance imaging in patientswithsarcoidosis. JACC Cardiovasc Imaging 2020; 13:1395–1405.
- Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, etal. Extensivelate gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 2014; 100:1165–1172.
- Yodogawa K, Seino Y, Ohara T, Takayama H, Katoh T, Mizuno K. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis: effect of corticosteroid therapy. Ann Non invasive Electrocardio l2011;16: 140–147.
837.Naruse Y, Sekiguchi Y, Nogami A, OkadaH, Yamauchi Y, MachinoT, et al. Systematic treatment approach to ventricular tachycardia in cardiac sarcoidosis. Circ Arrhythm Electrophysiol 2014;7: 407–413.
- SegawaM, Fukuda K, Nakano M, Kondo M, Satake H, Hirano M, etal. Time course and factors correlating with ventricular tachyarrhythmias after introduction of steroid therapy in cardiac sarcoidosis. Circ Arrhythm Electrophysiol 2016;9: e003353.
- Kumar S, BarbhaiyaC, NagashimaK, Choi E-K, EpsteinLM, JohnRM, et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate andoutcomes of catheter ablation. Circ ArrhythmElectrophysiol 2015;8: 87–93.
840.Muser D, Santangeli P, Liang JJ, Castro SA, Magnani S, Hayashi T, et al. Characterizationof theelectroanatomic substrate in cardiac sarcoidosis. JACC ClinElectrophysiol2018;4: 291–303.
- Jefic D, Joel B, Good E, Morady F, Rosman H, Knight B, etal. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiacsarcoidosis: report froma multi center registry. Heart Rhythm 2009; 6:1 89–195.
- Papageorgiou N, Providência R, Bronis K, Dechering DG, Srinivasan N, Eckardt L, etal. Catheter ablation for ventricular tachycardia in patients with cardiac sarcoidosis: a systematic review. EP Europace 2018; 20:682–691.
- Siontis KC, Santangeli P, Muser D, Marchlinski FE, Zeppenfeld K, Hoogendoorn JC, etal. Outcome sassociated with catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. JAMA Cardiol 2022; 7:175.
- Hoogendoorn JC, Venlet J, Out YNJ, Man S, Kumar S, Sramko M, etal. The precordial R’ wave: a novel discriminator between cardiac sarcoidosi sand arrhythmogenic right ventricularcardiomyopathy in patients presenting with ventricular tachycardia. Heart Rhythm 2021;18: 1539–1 547. 845. Bern C. Chagas’disease. N Engl J Med 2015; 373:456–466.
- Bocchi EA, Bestetti RB, Scanavacca MI, Cunha Neto E, Issa VS. Chronic Chagas heart disease management. J Am Coll Cardiol 2017; 70: 1510–1524.
- Nunes MCP, Dones W, Morillo CA, Encina JJ, Ribeiro AL. Chagas disease. J Am Coll Cardiol 2013; 62:767–776. 848.NunesMCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, et al. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American heart association. Circulation 2018; 138: e169–e209.
- Rassi A, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, etal. Development and validation of arisk score for predicting death in Chagas’heart disease. N Engl J Med 2006; 355:799–808.
- SenraT, Ianni BM, Costa ACP, Mady C, Martinelli – Filho M, Kalil-FilhoR, et al. Long-term prognostic value of myocardial fibrosis in patients with Chagas cardiomyopathy. JAmCollCardiol2018; 72:2577–2587.
- Rassi FM, Minohara L, Rassi A, Correia LCL, Marin-Neto JA, Rassi A, et al. Systematic review and meta-analysis of clinical outcome after implantable cardioverter – defibrillator therapy in patients with chagas heart disease. JACCC lin Electrophysiol 2019;5: 1213–1223.
- Cardinalli – Neto A, Bestetti RB, Cordeiro JA, Rodrigues VC. Predictors of all-cause mortality for patients with chronic Chagas’heart disease receiving implantablecar dioverterdefibrillatortherapy. J Cardiovasc Electrophysiol 2007; 18:1236–1240.
- Muratore CA, Batista Sa LA, Chiale PA, Eloy R, Tentori MC, Escudero J, et al. Implantable cardioverter defibrillators and chagas’disease: results of the ICD registry Latin America. Europace 2008;11: 164–168.
- Martinelli M, deSiqueira SF, Sternick EB, Rassi A, Costa R, Ramires JAF, et al. Long-termfollow-up of implantable cardioverter – defibrillator for secondary prevention in chagas’heartdisease. Am J Cardiol 2012; 110:1040–1045.
- Gali WL, Sarabanda AV, Baggio JM, Ferreira LG, Gomes GG, Marin-Neto JA, etal. Implantable cardioverter – defibrillators for treatment of sustained ventricularar rhythmias in patients with chagas’heart disease: comparison with acontrol group treated with amiodarone alone. Europace 2014; 16:674–680.
- Carmo AAL, de Sousa MR, Agudelo JF, Boersma E, Rocha MOC, Ribeiro ALP, etal. Implantable cardioverter-defibrillator in Chagas heart disease: a systematic review andmeta-analysisofobservationalstudies. Int J Cardiol2018; 267:88–93.
- Stein C, Migliavaca CB, Colpan iV, daRosa PR, Sganzerla D, Giordani NE, etal. Amiodarone for arrhythmia in patients with chagas disease: a systematic review and individual patient data meta-analysis. PLo S Negl Trop Dis 2018;12: e 0006742.
- Sosa E, Scanavacca M, D’Avila A, Piccioni J, Sanchez O, Velarde JL, etal. Endocardial andepicardial ablation Guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J CardiovascElectrophysiol 1998;9: 229–239.
- Soto-BecerraR, BazanV, Bautista W, Malavassi F, Altamar J, Ramirez JD, et al. Ventricular tachycardia in the setting of chagasic cardiomyopathy: use of voltage mapping to characterize endoepicardial nonischemic scar distribution. Circ ArrhythmElectrophysiol2017;10: e004950.
- Pisani CF, Romero J, Lara S, Hardy C, Chokr M, Sacilotto L, etal. Efficacy and safety of combined endocardial / epicardial catheter ablation for ventricular tachycardia in Chagas disease: a randomized controlled study. Heart Rhythm 2020;17: 1510–1518.
- Chizner MA, Pearle DL, deLeon AC. The natural history of aortic stenosis in adults. AmHeart J 1980; 99:419–424.
862.Delahaye JP, Gare JP, Viguier E, Delahaye F, De Gevigney G, Milon H. Natural history of severe mitral regurgitation. EurHeart J 1991; 12:5–9.
863.Groves P. Valve disease: surgery of valve disease: late results and late complications. Heart 2001; 86:715–721.
- Blackstone EH, Kirklin JW. Death and other time – related events after valve replacement. Circulation 1985;72: 753–767.
865.Urena M, Webb JG, Eltchaninoff H, Muñoz-García AJ, Bouleti C, Tamburino C, etal. Late cardiac death in patients under going transcatheter aorticvalve replacement: incidence andpredictors of advanced heart failure and sudden cardiacdeath. J Am Coll Cardiol 2015; 65:437–448.
- Yang F, Shah B, Iwai S, Markowitz SM, Lerman BB, Stein KM. ICD implantation and arrhythmia-freesurvival inpatients with depressedLVfunctionfollowingsurgery for valvular heart disease. Pacing Clin Electrophysiol 2008; 31:1419–1424.
- Valles AG, Khawaja FJ, Gersh BJ, Enriquez – Sarano M, Friedman PA, Park SJ, etal. Implantable cardioverter defibrillators in patients with valvular cardiomyopathy. J Cardiovasc Electrophysiol 2012; 23:1326–1332.
- Rodríguez – Mañero M, Barrio-López MT, Assi EA, Expósito-García V, Bertomeu-González V, Sánchez-Gómez JM, etal. Primary prevention of sudden death in patients with valvular cardiomyopathy. Rev Esp Cardiol (Engl Ed)2016; 69:272–278.
- Fischer – Rasokat U, Renker M, Liebetrau C, Weferling M, Rolf A, Hain A, et al. Long-termsurvival in patients with or without implantable cardioverter defibrillator after transcatheter aortic valve implantation. J Clin Med 2021; 10:2929.
- Nies RJ, Frerker C, Adam M, Kuhn E, Maur iV, Nettersheim FS, etal. Isthereabene fitof ICD treatment in patients with persistent severely reduced systolic left ventricular function after TAVI? Clin Res Cardiol 2022; 111: 492–501.
- Eckart RE, Hruczkowski TW, Tedrow UB, Koplan BA, Epstein LM, Stevenson WG. Sustained ventricular tachycardia associated with corrective valve surgery. Circulation 2007; 116:2005–2011.
- Liang JJ, Castro SA, Muser D, Briceno DF, Shirai Y, Enriquez A, et al. Electrophysiologic substrate, safety, procedural approaches, and outcomes of catheter ablation for ventricular tachycardia in patients after aortic valve replacement. JACCC lin Electrophysiol 2019; 5:28–38.
873.Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, andmolecularbasis. Circulation 2014; 129:2158–2170. 874. Nalliah CJ, Mahajan R, Elliott AD, Haqqan iH, Lau DH, Vohra JK, etal. Mitralvalve prolapse and sudden cardiac death: asystematic review and meta-analysis. Heart 2019; 105:144–151.
875.Nishimura RA, McGoon MD, Shub C, Miller FA, Ilstrup DM, Tajik AJ. Echocardiographically documented mitral – valve prolapse. Long – termfollow –up of 237 patients. N Eng l J Med 1985; 313:1305– 1309.
- Perazzolo Marra M, Basso C, DeLazzari M, Rizzo S, Cipriani A, Giorgi B, etal. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016; 9: e005030.
- Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez – Sarano M, Cetta F, etal. Malignant bileaflet mitral valve prolapse syndrome in patients with other wise idiopathic out -of- hospital cardiac arrest. J Am Col lCardiol 2013; 62:222–230.
- Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, etal. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol 2018; 72:823–834.
- Nordhues BD, SiontisKC, Scott CG, Nkomo VT, Ackerman MJ, Asirvatham SJ, etal. Bileaflet mitral valve prolapse and risk of ventricular dysrhythmias and death. J Cardiovasc Electrophysiol 2016; 27: 463–468. 880. Essayagh B, Sabbag A, Antoine C, Benfari G, YangL -T, Maalouf J, etal. Presentation andoutcomeof arrhythmicmitral valveprolapse. J AmColl Cardiol 2020;76: 637–649.
881.Narasimhan C, Jazayeri MR, SraJ, Dhala A, Deshpande S, Biehl M, etal. Ventricular tachycardia in valvular heart disease: facilitation of sustained bundle – branch reentry by valve surgery. Circulation 1997; 96:4307–4313.
- Moons P, BovijnL, Budts W, Belmans A, Gewillig M. Temporal trend sinsurvival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010; 122:2264–2272.
- Marelli AJ, Ionescu-Ittu R, Mackie AS, GuoL, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014; 130:749–756.
- Koyak Z, HarrisL, deGroot JR, Silversides CK, Oechslin EN, BoumaBJ, et al. Sudden cardiac death in adult congenital heart disease. Circulation 2012;126: 1944–1954.
- Gallego P, Gonzalez AE, Sanchez – Recalde A, PeinadoR, PoloL, Gomez-Rubin C, etal. Incidence and predictors of sudden cardiac arrest in adults with congenital heart defects repaired before adult life. Am J Cardiol 2012; 110: 109–117.
- Ghai A, Silversides C, Harris L, late after repair of tetralogy of Fallot. J Am Coll Cardiol 2002; 40:1675–1680.
- Khairy P, Landzberg MJ, Gatzoulis MA, Lucron H, Lambert J, Marçon F, etal. Value of programmed ventricular stimulation after tetralogy of fallot repair: a multicenter study. Circulation 2004; 109:1994–2000.
- Kapel GFL, Sacher F, Dekkers OM, Watanabe M, Blom NA, ThamboJ-B, etal. Arrhythmogenic anatomical isthmuses identified by electroanatomical mapping are the substrate for ventricular tachycardia inrepairedtetralogyof Fallot. Eur Heart J2017;38: 268–276.
- Atallah J, Gonzalez Corcia MC, Walsh EP. Participating members of the pediatric and congenital electrophysiology society. Ventricular arrhythmia and life threatening events in patients with repaired tetralogy of Fallot. Am J Cardiol 2020; 132:126–132.
- Kammeraad JAE, van Deurzen CHM, Sreeram N, Bink – Boelkens MTE, Ottenkamp J, Helbing WA, etal. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol 2004; 44:1095–1102.
- Schwerzmann M, Salehian O, HarrisL, Siu SC, Williams WG, Webb GD, etal. Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J 2009; 30:1873–1879.
892.Miyazaki A, Sakaguchi H, Ohuchi H, Matsuoka M, Komori A, Yamamoto T, etal. Efficacy of hemodynamic – based management of tachyarrhythmia after repair of tetralogy of Fallot. Circ J 2012;76: 2855–2862.
- Harrison DA, Harris L, Siu SC, MacLoghlin CJ, Connelly MS, Webb GD, et al. Sustained ventricular tachycardia in adult patients lateafterrepairof tetralogyof Fallot. J Am Coll Cardiol 1997; 30: 1368–1373.
- SabateRotes A, Connolly HM, Warnes CA, Ammash NM, Phillips SD, Dearani JA, etal. Ventricular arrhythmia risk stratification in patients with tetralogy of Fallotat the time of pulmonary valve replacement. Circ Arrhythm Electrophysiol 2015; 8: 110–116.
- Khairy P, HarrisL, Landzberg MJ, Fernandes SM, Barlow A, Mercier L-A, et al. Sudden death and defibrillators in transposition of the great arteries with intra – atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol 2008; 1:250–257.
- Roca – LuqueI, Rivas Gándara N, Dos SubiràL, Francisco Pascual J, Pérez – Rodon J, Pijuan Domenech A, etal. Intra-atrialre-entranttachycardia in patients with congenital heart disease: factors associated with disease severity. Europace 2018; 20: 1343–1351.
- Zeppenfeld K, Schalij MJ, Bartelings MM, Tedrow UB, Koplan BA, Soejima K, etal. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of thecritical rightventricular isthmus. Circulation 2007;116: 2241–2252.
- Kriebe lT, Sau lJP, Schneider H, Sigler M, Paul T. Noncontact mapping and radio frequency catheter ablation of fast and hemodynamically unstable ventricular tachycardia after surgical repair of tetralogy of Fallot. J AmColl Cardiol 2007;50: 2162–2168.
- vanZylM, KapaS, Padmanabhan D, Chen FC, Mulpuru SK, Packer DL, et al. Mechanism and outcomes of catheter ablation for ventricular tachycardia in adults with repaired congenital heart disease. Heart Rhythm 2016; 13:1449–1454.
- Laredo M, Frank R, Waintraub X, Gandjbakhch E, Iserin L, HascoëtS, etal. Ten-year outcomes of monomorphic ventricular tachycardia catheter ablation in repaired tetralogy of Fallot. Arch Cardiovasc Dis 2017; 110:292–302.
- Kape lGFL, Reichlin T, Wijnmaalen AP, Piers SRD, Holman ER, Tedrow UB, etal. Re-entry using anatomically determined isthmuses: acurable ventricular tachycardia in repaired congenital heart disease. Circ Arrhythm Electrophysiol 2015; 8: 102–109.